Sodium 8-(2-hydroxylbenzamido)caprylate and preparation method therefor
A technology of hydroxybenzamide and sodium octanoate, which is applied in the preparation of carboxylic acid amides, chemical instruments and methods, and the preparation of organic compounds, and can solve the problems of difficult removal of impurities, low yield, and unsuitability for industrial production. To achieve the effect of increasing the yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
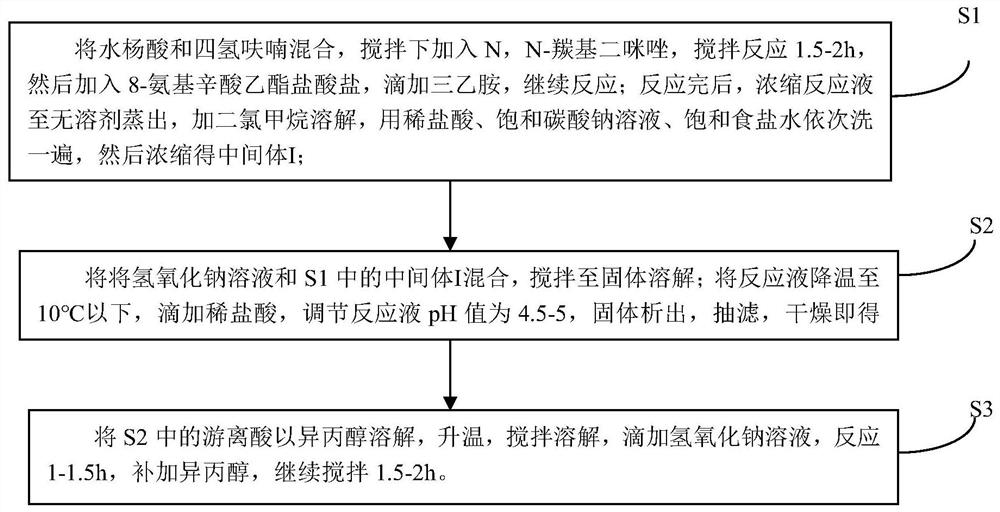
[0037] A kind of preparation method of 8-(2-hydroxybenzamido) octanoic acid sodium, comprises the following steps:
[0038] S1: Weigh 20.0628g of salicylic acid into a 500mL reaction flask, add 100ml of tetrahydrofuran, slowly add 23.4132g of N,N-carbonyldiimidazole under stirring, a large amount of gas is generated. After the addition, continue to stir at room temperature for 1.5h. Then 32.4143g of ethyl 8-aminocaprylate hydrochloride was added, and then 24ml of triethylamine was added dropwise, and the reaction was continued for 28h at 20°C with stirring. The reaction solution was concentrated to dryness, that is, when no solvent was evaporated, 200 mL of dichloromethane was added to dissolve, and then washed with 1 mol / L dilute hydrochloric acid, saturated sodium bicarbonate solution, and saturated brine, and the organic phase was concentrated to obtain 45.8 g of intermediate I. Yield 102.9% (wet weight yield).
[0039] S2: Add 460 mL of 2 mol / L sodium hydroxide solution ...
Embodiment 2
[0047] S1: Weigh 5g of salicylic acid into a 100mL reaction flask, add 20ml of tetrahydrofuran, slowly add 5.85g of N,N-carbonyldiimidazole under stirring, a large amount of gas is generated. After the addition, continue to stir at room temperature for 2h. Then 8.1g of ethyl 8-aminocaprylate hydrochloride was added, and then 7.5ml of triethylamine was added dropwise, and the reaction was continued to stir at 23°C for 22h. The reaction solution was concentrated to dryness, that is, when no solvent was evaporated, 50 mL of dichloromethane was added to dissolve, and then washed with 1 mol / L dilute hydrochloric acid, saturated sodium bicarbonate solution, and saturated brine, and the organic phase was concentrated to obtain 11.3 g of intermediate I. Yield 101.6% (wet weight yield).
[0048] S2: Add 200 mL of 2 mol / L sodium hydroxide solution to the concentrated bottle containing intermediate I in S1, and stir the reaction until all solids are dissolved at room temperature of 25°C...
Embodiment 3
[0051] S1: Weigh 200g of salicylic acid into a 4L reaction flask, add 1L of tetrahydrofuran, slowly add 234N,N-carbonyldiimidazole under stirring, a large amount of gas is generated. After the addition, continue to stir at room temperature for 2h. Then, 324g of ethyl 8-aminocaprylate hydrochloride was added, and then 300ml of triethylamine was added dropwise, and the stirring reaction was continued at 30°C for 30h. The reaction solution was concentrated to dryness, that is, when no solvent was evaporated, 2L of dichloromethane was added to dissolve, and then washed with 1mol / L dilute hydrochloric acid, saturated sodium bicarbonate solution, and saturated brine respectively, and the organic phase was concentrated to obtain 447g of intermediate I. Yield 100.5% (wet weight yield).
[0052] S2: Add 5L of 2mol / L sodium hydroxide solution to the concentrated bottle containing intermediate I in S1, and stir the reaction until all solids are dissolved at room temperature of 30°C. Th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com