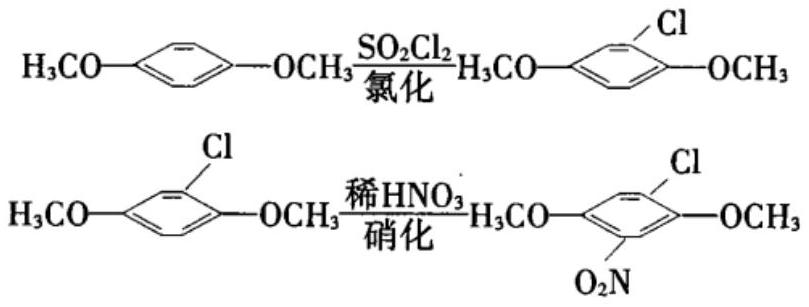
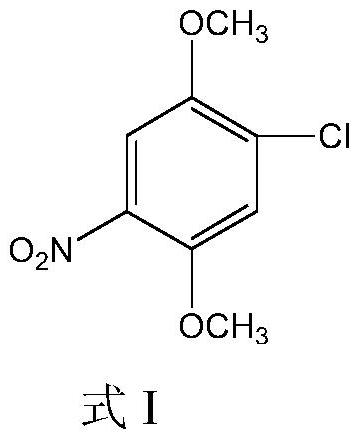
Method for synthesizing 4-chloro-2, 5-dimethoxynitrobenzene by using microreactor
A technology of dimethoxynitrobenzene and dimethoxychlorobenzene, applied in the field of preparation of 4-chloro-2,5-dimethoxynitrobenzene, which can solve the problem of uncontrollable reaction heat release and low production efficiency , Reaction with large liquid holding capacity and other problems, to achieve the effect of small liquid holding capacity, short residence time and low reaction risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Using 2,5-dimethoxychlorobenzene and nitric acid as raw materials, 4-chloro-2,5-dimethoxynitrobenzene was synthesized by microreactor. Wherein the solvent for selection is ethylene dichloride, the concentration of 2,5-dimethoxychlorobenzene (chloride) is 30%, the concentration of nitric acid is 70%, the feed ratio is 1.3 equivalents, and the feed temperature is 30°C. The reaction temperature is 60°C, the reaction residence time is 60s, and the liquid holding capacity of the microreactor is 200mL. The summary is shown in Table 1:
[0023] Summary of reaction conditions in table 1 embodiment 1
[0024]
Embodiment 2~25
[0026] Solvent among the embodiment 1, nitric acid concentration, equivalent, and chloride concentration, feed temperature, temperature of reaction and residence time are changed 1 or more variable, the impact on reaction result is as shown in table 2 below:
[0027] Table 2 Summary of different reaction conditions and reaction results
[0028]
[0029] It can be seen from the above that when chlorinated alkanes are used as solvents, the reaction progresses to the greatest extent, and the residual amount of chlorides is the lowest. In the case of the same solvent, the concentration of nitric acid and the reaction temperature have the greatest influence on the progress of the reaction. Taking dichloroethane as the solvent as an example, when the concentration of nitric acid is 70-80% and the reaction temperature is 60-80°C, the residual amount of chloride is the lowest.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com