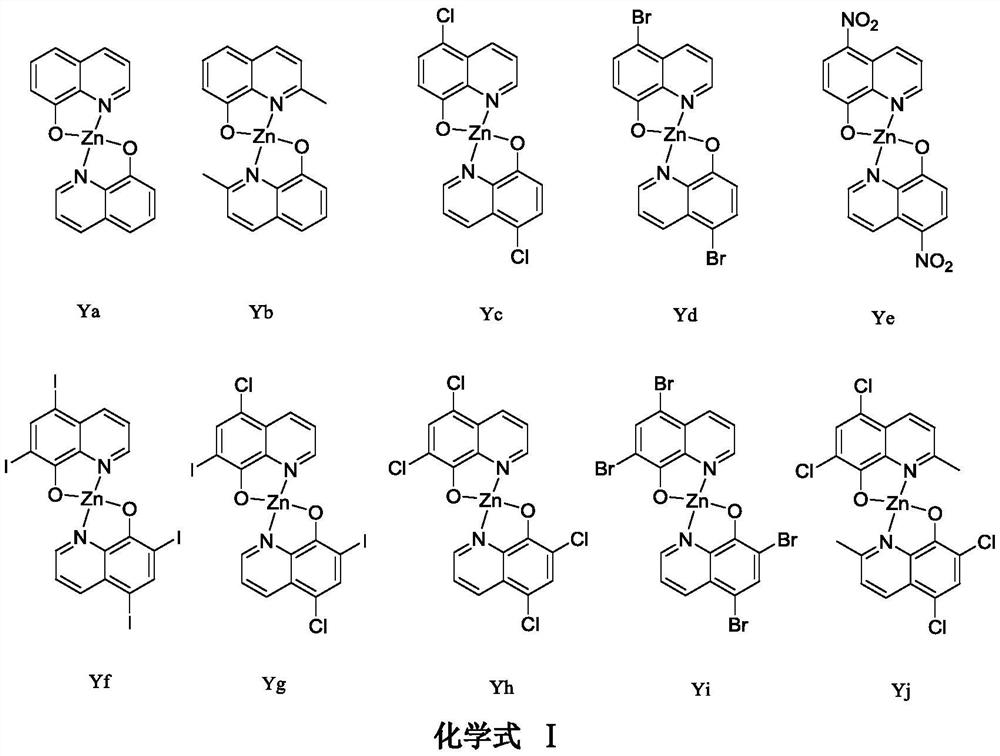
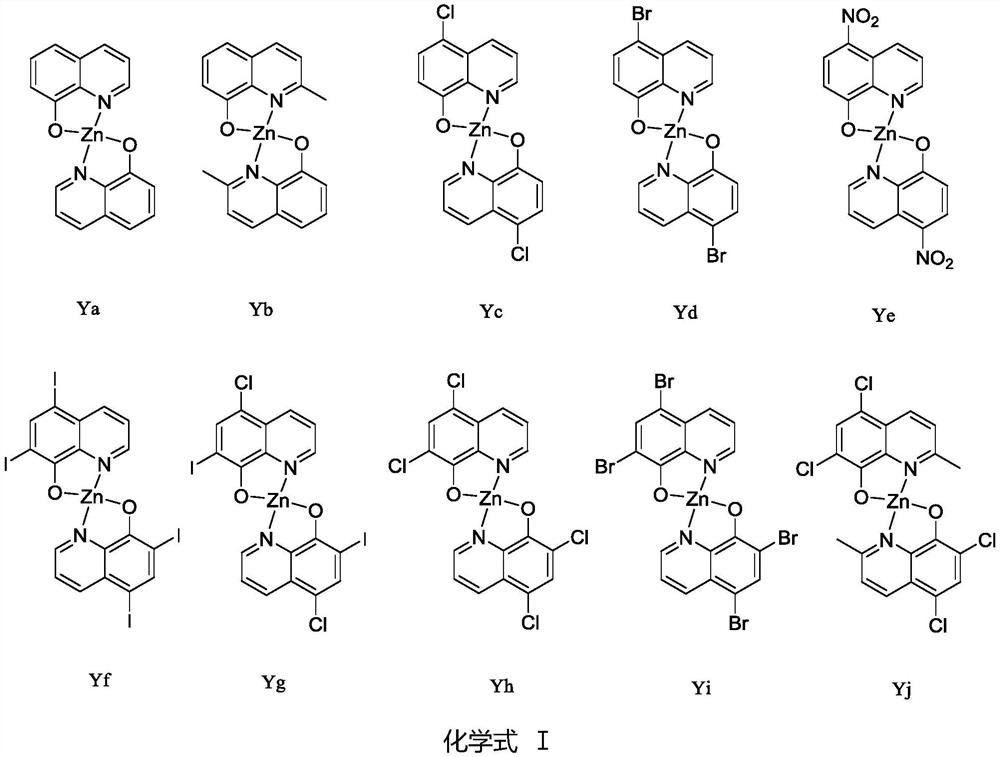
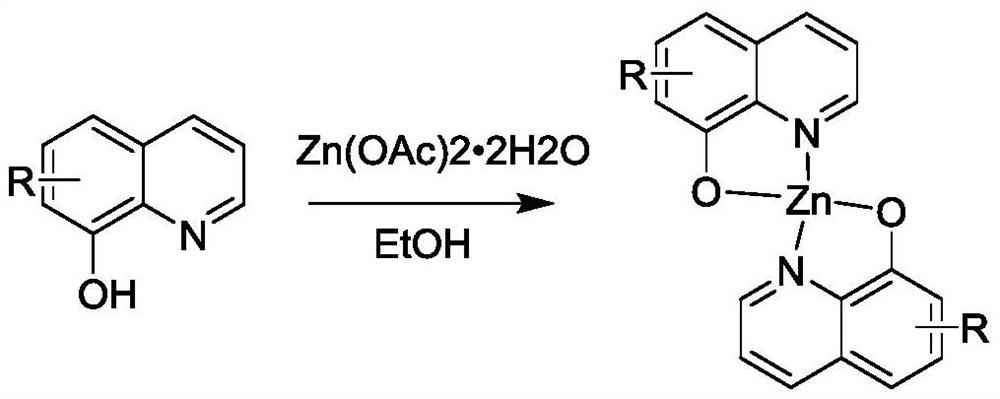
Preparation of 8-hydroxyquinoline complexes and application of 8-hydroxyquinoline complexes in prevention and treatment of plant diseases
A technology of hydroxyquinoline zinc and complexes, which is applied in plant growth regulators, botany equipment and methods, chemicals for biological control, etc., and can solve problems such as increased resistance of pathogenic bacteria, environmental pollution, and excessive drug residues , to achieve the effects of high bactericidal activity, high product purity and simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Synthesis of 8-hydroxyquinoline zinc complex (Ya)
[0019]
[0020] For the synthesis method, please refer to the reference method: Polyhedron 154(2018) 65-76. The specific synthesis operation is as follows: Synthesis of 8-hydroxyquinoline zinc complex (Ya) (Chemical formula III): Weigh 8-hydroxyquinoline (10mmol) Place it in a 100mL three-necked flask equipped with a condenser and a magnetic stirrer, dissolve it with 50mL of absolute ethanol, heat the solution to 70℃ in a water bath, weigh out zinc acetate dihydrate (5mmol) and dissolve it in 10mL of deionized water. The zinc acetate solution was slowly added dropwise (about 0.5h) to the ethanol solution of 8-hydroxyquinoline in a constant pressure dropping funnel, the reaction was magnetically stirred for 2h, and the reaction was stopped. A large amount of yellow-green solid precipitated out, filtered with suction, and the crude product was used separately Repeated washing with absolute ethanol and deionized water, vacuu...
Embodiment 2
[0023] Synthesis of 2-methyl-8-hydroxyquinoline zinc complex (Yb):
[0024] The experimental procedure is the same as in Example 1, except that 2-methyl-8-hydroxyquinoline is used instead of 8-hydroxyquinoline.
[0025]
[0026] Yb yellow-green solid, yield 97.6%; 1 H NMR(400MHz,DMSO-d6)δ8.28(d,J=8.4Hz,2H), 7.49(d,J=8.4Hz,2H), 7.28(t,J=7.9Hz,2H), 6.95-6.86 (m,2H),6.75-6.66(m,2H),2.94(s,6H).FTIR(KBr)ν / cm-1:3050.6(Ar-H);3036.0(-CH3);1605.2,1591.3,1567.6 , 1507.2 (quinoline ring skeleton vibration); 1462.4, 1377.7 (-CH3); 832.5, 750.0 (Ar-H); 509.7, 468.6 (Zn-N, Zn-O).
Embodiment 3
[0028] Synthesis of 5-chloro-8-hydroxyquinoline zinc complex (Yc):
[0029] The experimental procedure is the same as in Example 1, except that 5-chloro-8-hydroxyquinoline is used instead of 8-hydroxyquinoline.
[0030]
[0031] Yc yellow solid, yield 85.0%; 1 H NMR(400MHz,DMSO-d6)δ8.97(m,J=9.0Hz,2H),8.83(m,2H),7.69(m,2H),7.59(d,J=9.5Hz,2H),6.61 (d,2H).FTIR(KBr)ν / cm-1:3046.7(Ar-H);1595.2,1572.5,1491.6,1455.9(quinoline ring skeleton vibration);1380.9(CO);782.6,736.9(Ar-H ); 531.0, 499.4 (Zn-N, Zn-O).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com