Double-blind drug clinical test method and system
A technology of clinical trials and drugs, applied in the direction of drugs or prescriptions, can solve problems such as drug waste, achieve the effects of saving drugs, solving drug waste, and optimizing the distribution process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
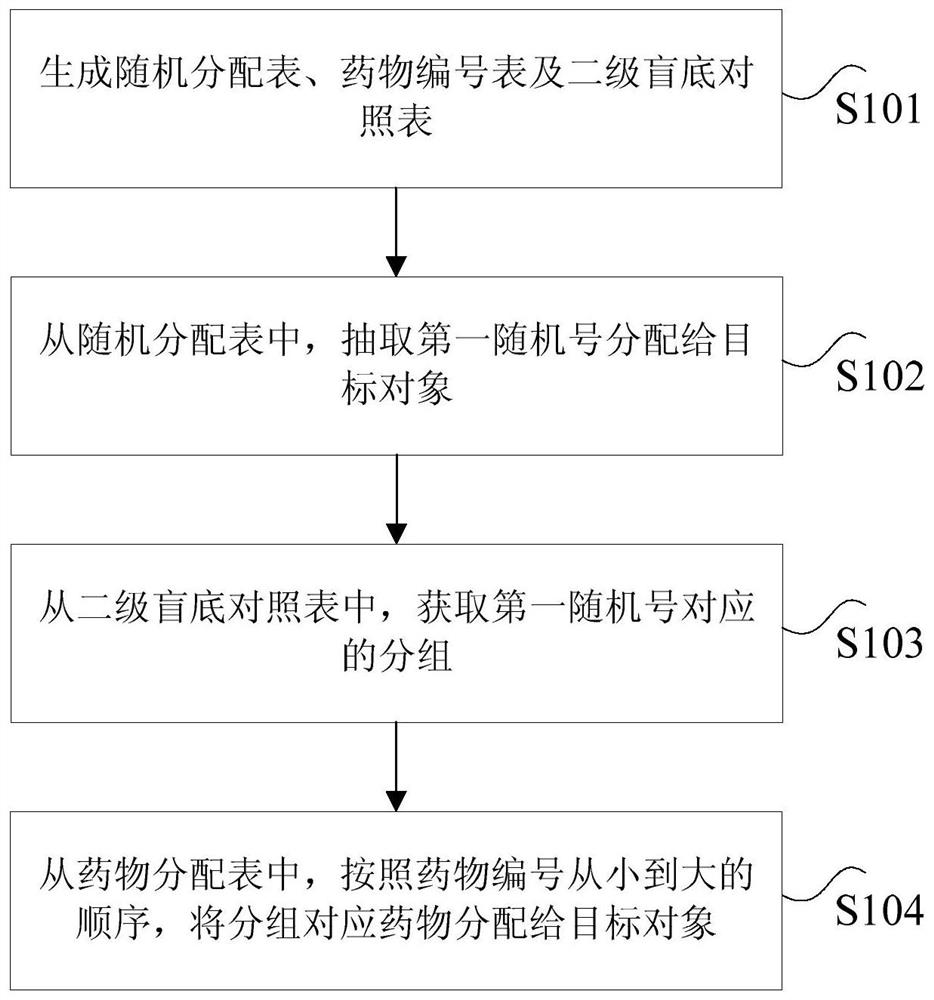
[0026] According to an embodiment of the present invention, a method embodiment of a double-blind drug clinical trial method is provided. It should be noted that the steps shown in the flow chart of the accompanying drawings can be executed in a computer system such as a set of computer-executable instructions , and, although a logical order is shown in the flowcharts, in some cases the steps shown or described may be performed in an order different from that shown or described herein.
[0027] figure 1 It is the double-blind drug clinical trial method according to the embodiment of the present invention, such as figure 1 As shown, the method includes the following steps:
[0028] Step S101, generating a random allocation table, a drug number table, and a secondary blind comparison table.
[0029] In the above step S101 of the present application, at the beginning of the test, firstly, two random control tables (ie, random allocation table and drug number table) and one seco...
Embodiment 2
[0049] According to an embodiment of the present invention, a double-blind drug clinical trial system is also provided, such as figure 2 As shown, the double-blind drug clinical trial system includes: an input unit 201, which is used to receive the basic information of the target object; a screening unit 202, which is used to screen the target object based on the basic information and preset inclusion and exclusion criteria; a processing unit 203, for extracting the first random number from the pre-generated random allocation table and assigning it to the target object; obtaining the group corresponding to the first random number from the pre-generated secondary blind comparison table; In the table, according to the order of drug numbers from small to large, the drugs corresponding to the groups are assigned to the target objects.
[0050] Optionally, the random allocation table includes random numbers, group numbers, blocks and random numbers.
[0051]Optionally, the drug n...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com