Signal peptide for promoting protein extracellular expression
A signal peptide and protein technology, applied in the fields of genetic engineering and enzyme engineering, can solve the problems of changing catalytic properties and limited improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
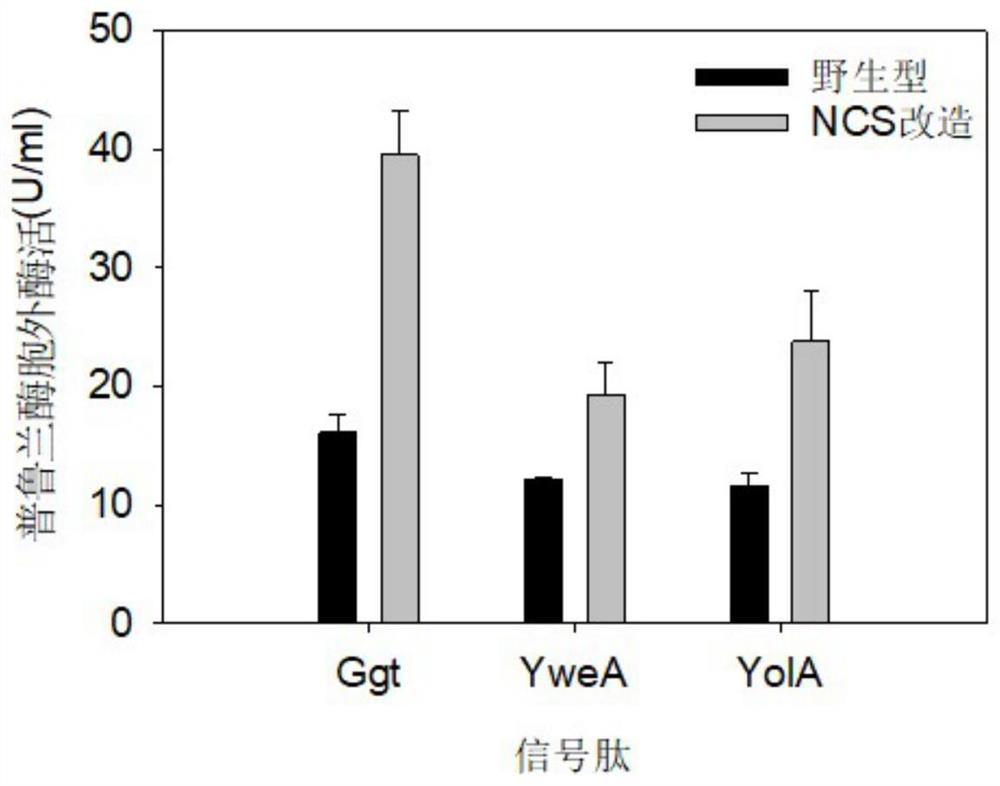
[0049] Example 1: Construction of Ggt signal peptide NCS synonymous mutation library
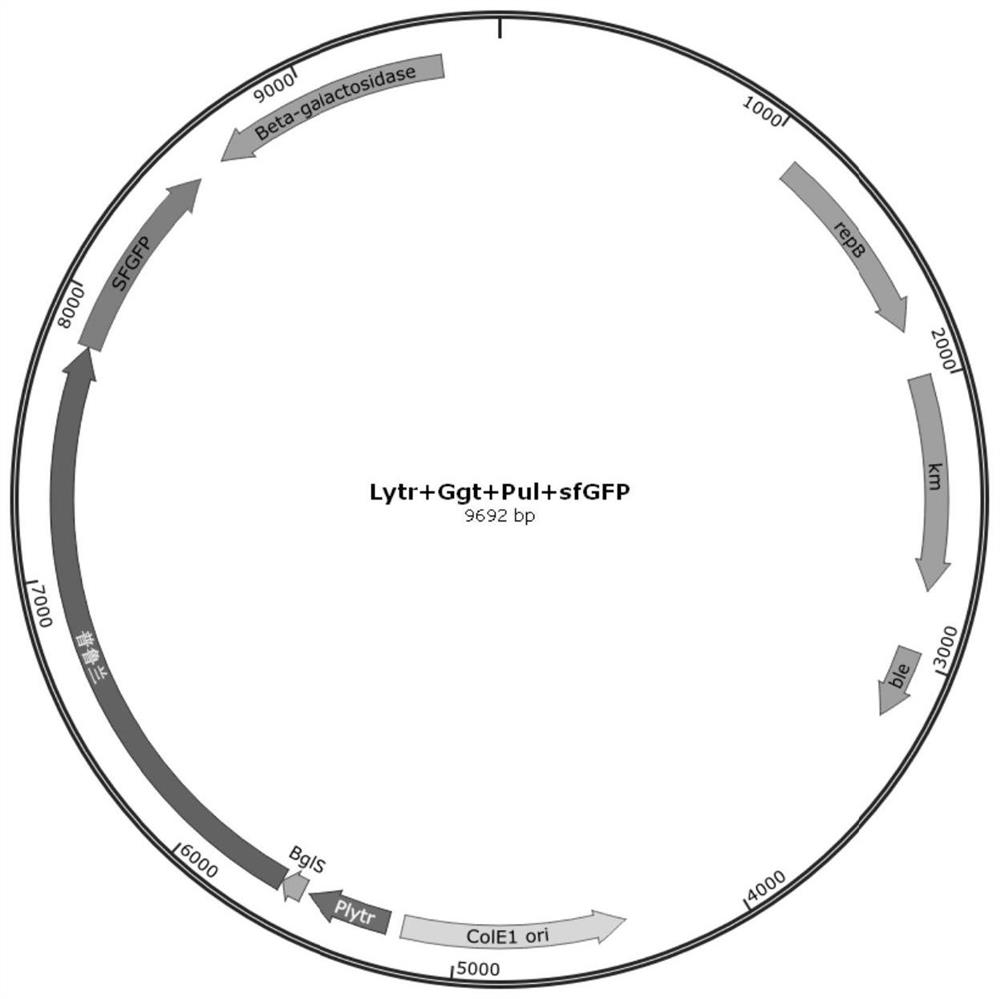
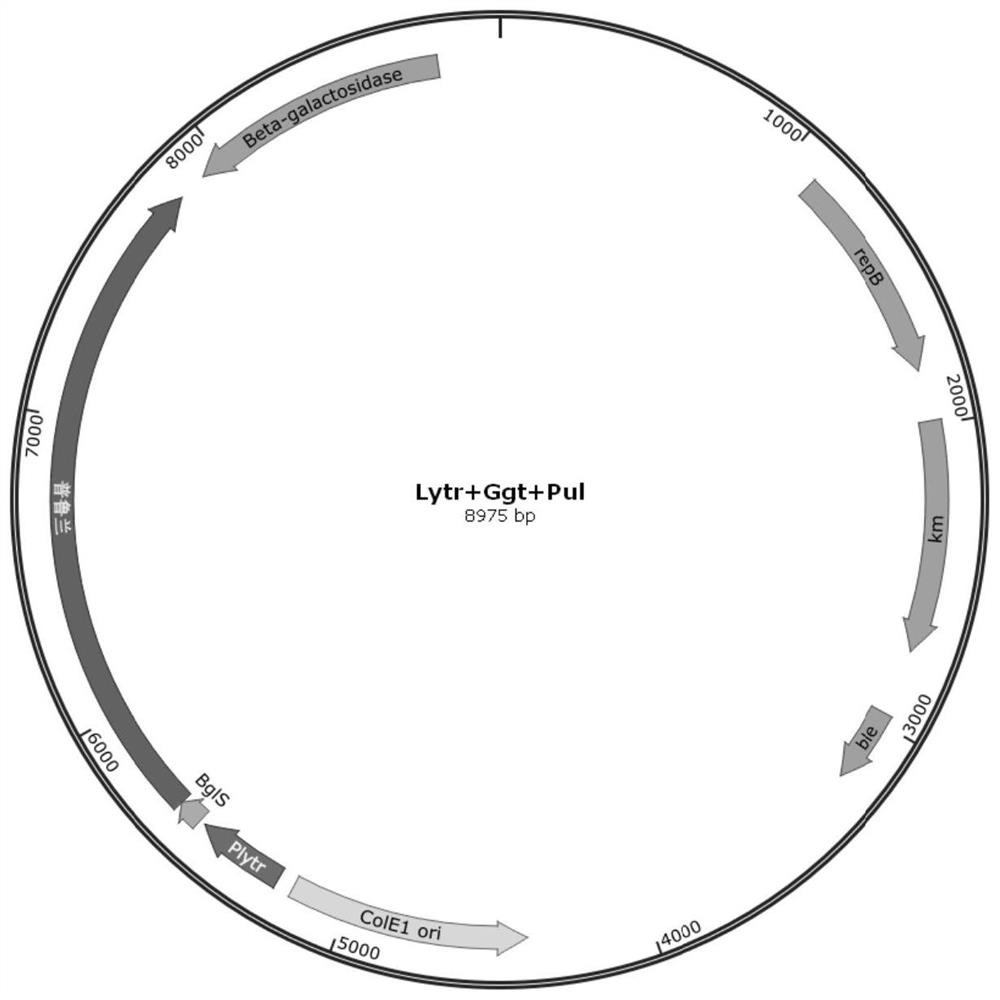
[0050]In order to promote the high-efficiency expression of pullulanase, the Bacillus subtilis transcription LytR promoter (shown in SEQ ID NO.4 for sequence information) with the highest endogenous transcription level characterized by previous laboratories was passed through a one-step cloning kit (purchased Connected to the P43NMK plasmid from Nanjing Nuoweizan Biotechnology Co., Ltd. to construct a recombinant plasmid, transfer the recombinant plasmid into E.coli JM109, and spread the bacterial solution on an LB plate containing 50 μg / mL ampicillin resistance , cultivated at 37°C until a single clone was grown, and the single clone was picked out and verified by plasmid sequencing after culture to obtain the plasmid P43NMK-LytR; using the same one-step cloning method, the pullulanase gene (nucleotide sequence such as SEQ ID NO .6) was fused to the downstream of LytR by a one-step cloning ...
Embodiment 2
[0052] Example 2: Construction of YweA signal peptide NCS synonymous mutation library
[0053] The specific embodiment is the same as in Example 1, the difference is that after obtaining P43NMK-LytR-Pul, the sfGFP fluorescent protein is connected to the C-terminus of pullulanase by a one-step cloning method, and the YweA signal peptide wild-type (nucleotide The sequence is shown in SEQ ID NO.9) was connected to the N-terminal of pullulanase to construct the recombinant plasmid P43NMK-YweA-Pul.
[0054] Using P43NMK-YweA-Pul as a template, using degenerate primers (nucleotide sequences shown in SEQ ID NO.11 and SEQ ID NO.13), PCR obtained a synonymous mutation library of the first 30 bases of the N-terminal of YweA ( Synonymous mutation recombinant plasmid), that is, the first 30 amino acid sequences remain unchanged, and the nucleotide sequence is changed.
Embodiment 3
[0055] Example 3: Construction of YolA signal peptide NCS synonymous mutation library
[0056] The specific embodiment is the same as in Example 1, the difference is that after obtaining P43NMK-LytR-Pul, a one-step cloning method is used to connect the sfGFP fluorescent protein to the C-terminus of pullulanase, and the YolA signal peptide wild-type (nucleotide The sequence is shown in SEQ ID NO.10) was connected to the N-terminal of pullulanase to construct the recombinant plasmid P43NMK-YolA-Pul.
[0057] Using P43NMK-YolA-Pul as a template, using degenerate primers (nucleotide sequences shown in SEQ ID NO.11 and SEQ ID NO.14), PCR obtained a synonymous mutation library of the first 30 bases of the N-terminal of YolA ( Synonymous mutation recombinant plasmid), that is, the first 30 amino acid sequences remain unchanged, and the nucleotide sequence is changed.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com