Novel BCL-2/BCL-XL inhibitor, pharmaceutical composition and application
A technology for BCL-2 and inhibitors, which is applied in the field of drugs for synthesizing Bcl-2/BCL-XL anti-apoptotic protein inhibitors, and can solve the problems of increasing drug doses, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Synthesis of compound (I)
[0047] The synthetic process is shown in reaction scheme 1
[0048]
[0049] Refer to the relevant intermediate 5a of the reference in the prior art ("BCL-2 Selective Inhibitor--Synthetic Process Improvement of ABT-199" Xu Yunlei et al., "Synthetic Chemistry", Volume 23, Issue 11, 1063-1067) and the synthesis of intermediate 9a. Intermediates 1a, 3a, 4a, 5a, 7a, 8a, and 9a can refer to prior art references ("BCL-2 Selective Inhibitor--Synthetic Process Improvement of ABT-199" Xu Yunlei et al., "Synthetic Chemistry", Volume 23 Issue 11, 1063-1067) Synthesis.
[0050] Synthesis of Intermediate Compound 1a
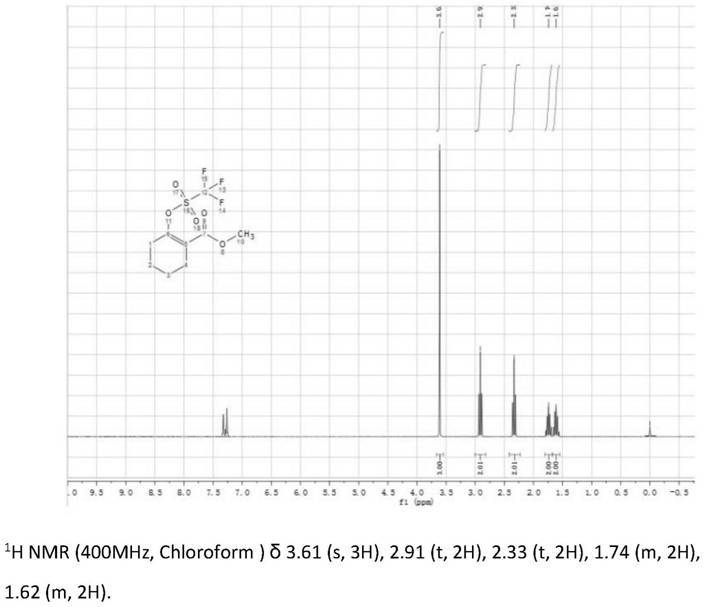
[0051] Add 333g (832mmol) of sodium hydride, 186g (2.06mol) of dimethyl carbonate and 880mL of tetrahydrofuran into a round-bottomed flask equipped with a magnetic stirrer, a condenser and an inert gas source, and stir to dissolve; add the raw materials dropwise at (60°C) Compound 0a39g (396mmol) was dissolved in 440mL of tetrahydrofu...
Embodiment 2
[0073] The synthesis of embodiment 2 compound II
[0074] The synthetic process is shown in reaction scheme 2
[0075]
[0076] The synthesis of intermediate compound 11a is shown in Example 1.
[0077] Synthesis of Compound II
[0078] Add 50 mL of an aqueous solution containing 0.8 g (20 mmol) of sodium hydroxide in a round bottom flask equipped with a magnetic stirrer, then add 3.72 g (10 mmol) of compound 11a and compound 5b 2.55g (10mmol), stirred overnight at room temperature, and monitored the reaction by TLC. Extract with dichloromethane (2×100mL), combine the organic phases, wash with saturated brine successively, dry over anhydrous sodium sulfate, concentrate under reduced pressure, and the residue is subjected to silica gel column chromatography [eluent: petroleum ether / ethyl acetate=5 / 1] to obtain (compound II) 4.72g, yield 80%. Its NMR see attached Figure 9 . LCMS [M+H]: 591.2. 1 H NMR (400MHz, Chloroform) δ8.28(d,1H), 7.79(s,1H),7.59(m,1H),7.41-7.45(...
Embodiment 3
[0079] The synthesis of embodiment 3 compound III
[0080] The synthetic process is shown in reaction scheme 3
[0081]
[0082] The synthesis of intermediate compound 11a is shown in Example 1.
[0083] Synthesis of Compound III
[0084] Add 50 mL of an aqueous solution containing 0.8 g (20 mmol) of sodium hydroxide in a round bottom flask equipped with a magnetic stirrer, then add 3.72 g (10 mmol) of compound 11a and compound 5c 2.15 g (10 mmol), stirred overnight at room temperature, and monitored the reaction by TLC. Extract with dichloromethane (2×100mL), combine the organic phases, wash with saturated brine successively, dry over anhydrous sodium sulfate, concentrate under reduced pressure, and the residue is subjected to silica gel column chromatography [eluent: petroleum ether / ethyl acetate=5 / 1] to obtain (compound III) 4.40g, yield 80%. Its NMR see attached Figure 10 . LCMS [M+H]: 551.2. 1 H NMR (400MHz, Chloroform) δ8.29(d,1H), 7.81(s,1H),7.58(m,1H),7.41...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com