Use of method for purifying, isolating and analyzing atypical circulating tumor cells and use of atypical circulating tumor cells
A tumor cell, purification and separation technology, applied in the field of atypical circulating tumor cells, which can solve problems such as omission
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1. Whole blood sample processing and CD45-negative and EpCAM-negative cell counting methods
[0028] In this example, the experiments were approved by the Institutional Review Board of the Chang Gung Memorial Hospital. All blood sample donors obtained informed consent (approval number: 201601081B0), and all methods were carried out in accordance with relevant guidelines for clinical trials.
[0029] First, 4 mL of a whole blood sample derived from one individual is provided, and then blood cells are removed from the 4 mL of the whole blood sample. Use red blood cell lysate, 1L of red blood cell lysate contains 8.26g of NH 4 Cl, 1.19g of NaHCO 3 , 200 μL of 0.5M EDTA (ethylenediaminetetraacetic acid), pH 8, with a final pH of 7.3. Among them, the volume ratio of the whole blood sample to the red blood cell lysate is 1:10, and the reaction does not exceed 10 minutes, and the supernatant is removed by centrifugation after the reaction. Next, centrifuge at a rot...
Embodiment 2
[0032] Example 2. Specificity and Sensitivity Tests of CD45 Negative and EpCAM Negative Cells for Differentiation of Cancer Patients
[0033] In this example, the experiment was approved by the human experiment ethics department of Chang Gung Memorial Hospital. All blood sample donors obtained informed consent (approval number: 201601081B0), and all methods were carried out in accordance with relevant guidelines for clinical trials.
[0034] First, CD45-negative and EpCAM-negative atypical circulating tumor cells and their number information were obtained according to the operation procedure described in Example 1 above. Next, use the receiver operating characteristic curve (ROC curve) of the analysis population to set the cut-off value (cut-off value), where the cut-off value is the need to diagnose cancer, evaluate cancer prognosis, track and monitor cancer or evaluate cancer treatment effectiveness The average, median, or a value obtained by receiver operating characterist...
Embodiment 3
[0041] Example 3. The clinical significance of the number of typical circulating tumor cells and the number of CD45-negative and EpCAM-negative cells
[0042] In this example, the experiment was approved by the human experiment ethics department of Chang Gung Memorial Hospital. All blood sample donors obtained informed consent (approval number: 201601081B0), and all methods were carried out in accordance with relevant guidelines for clinical trials.
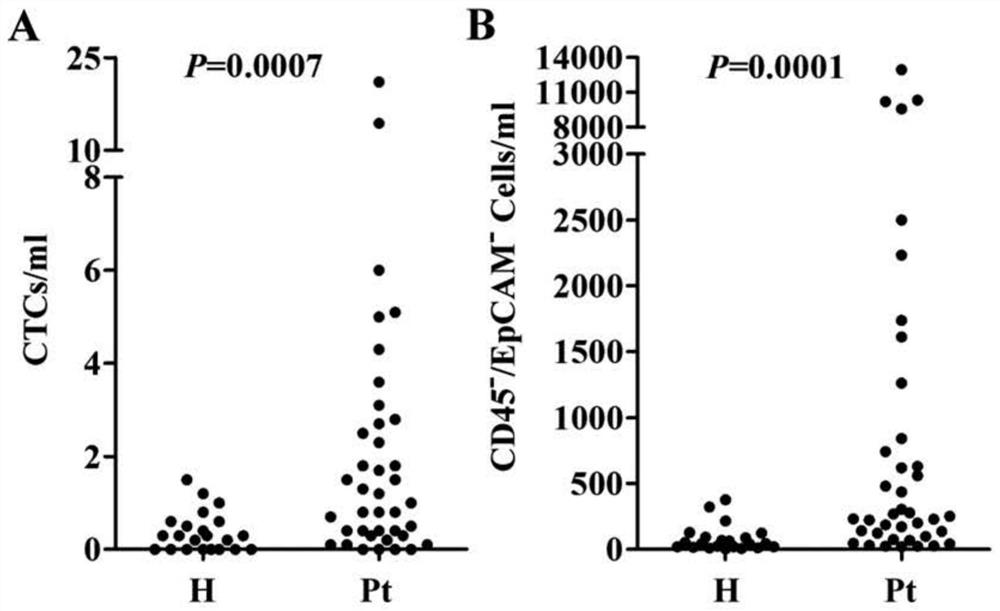
[0043] First, CD45-negative and EpCAM-negative atypical circulating tumor cells and their number information were obtained according to the operation procedure described in Example 1 above. Then, whether there is a difference in the average number of cells between healthy subjects and cancer patients by statistical analysis method, wherein the statistical method is Mann-Whitney U test (Mann-Whitney U test), P value less than 0.05 is considered Significant difference.
[0044] figure 2 Typical circulating tumor cells (A) and C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com