Primer probe combination and kit for detecting five important arthropod/ odonto-mediated viruses and application of primer probe combination and kit
A primer probe and virus detection technology, which is applied in the direction of recombinant DNA technology, microorganism-based methods, microorganism measurement/inspection, etc., can solve the problems of high reagent price, long detection time, and inability to achieve instant detection, etc., and achieve good application Effects of foreground, high sensitivity, and specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] Example 1 Repeatability Detection
[0090] The positive control of each virus diluted 10 times was used as the sample to be tested for repeated detection, and the detection was repeated 3 times.
[0091]1. Nucleic acid extraction (used with Hs480 heating centrifuge)
[0092] 1) Take 50 μl of the sample and add 5 μl FastLyse L4, shake and mix;
[0093] 2) Place the mixed sample in the HS480 heating centrifuge machine, heat at 95°C for 2min, and centrifuge at 5000rpm for 2min;
[0094] 3) After centrifugation, take the supernatant as the sample RNA, numbered;
[0095] 4) The positive control, negative control and samples to be tested are processed synchronously.
[0096] 2. Preparation of PCR reagents
[0097] 1) Prepare the PCR reaction solution: pre-mix the ultra-fast PCR buffer1 (68 μl) and the ultra-fast PCR enzyme system (17 μl) (2 more than the actual number of reaction tubes, to avoid loss during sample addition, and to avoid air bubbles during mixing);
[009...
Embodiment 2
[0115] Embodiment 2 specific detection
[0116] Each virus positive control was used as the sample to be tested for specific detection, and the detection method was the same as in Example 1.
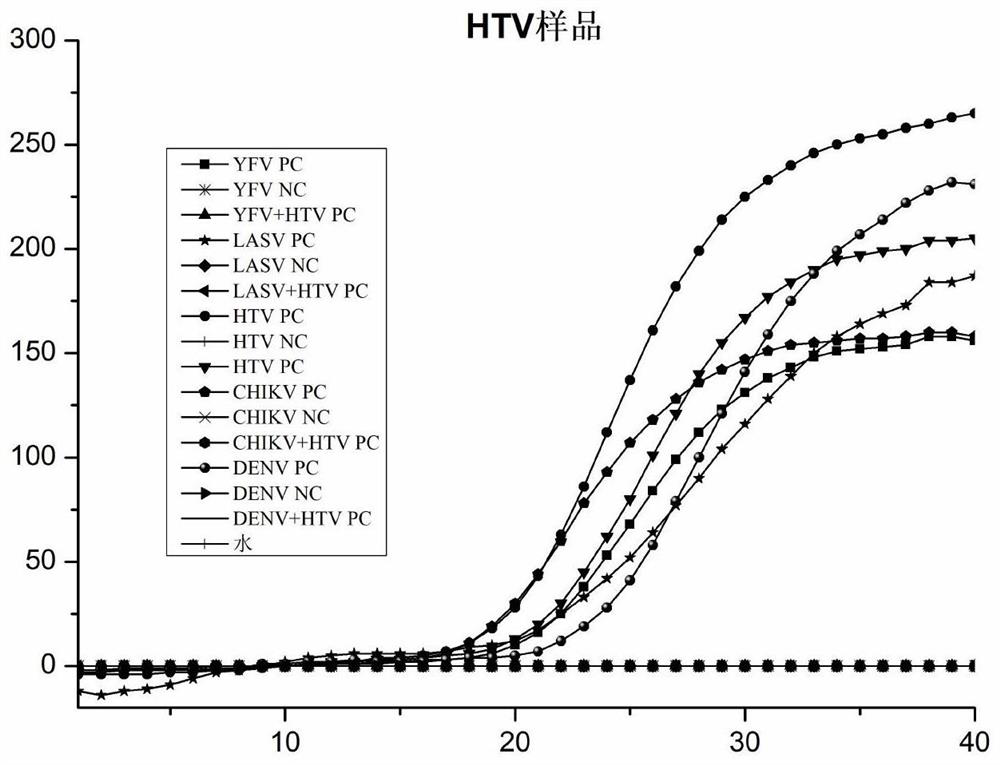
[0117] Result: Each virus positive control sample was only positive under the corresponding probe, and the rest were negative, with good specificity. The results were as follows: Figure 1 to Figure 5 shown.
Embodiment 3
[0118] Example 3 Sensitivity Detection
[0119] Each virus positive control in the kit (concentration is 1 × 10 5 copies / ml) was serially diluted to 1×10 4 copies / ml, 1×10 3 copies / ml, 1×10 2 copies / ml and 1×10 1 Copies / ml, after preparation, use it as the sample to be tested for sensitivity testing with the corresponding kit.
[0120] Concrete implementation steps are with embodiment 1.
[0121] Results: The detection limits of YFV, LASV, HTV, CHIKV and DENV were not higher than 1×10 1 copies / ml, the result is as Figure 6 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com