Prefilled syringe and method of manufacture
A syringe and pre-filled technology, applied in syringes, ampoule syringes, infusion sets, etc., can solve problems such as slow release of lidocaine, and achieve the effect of excellent dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1: Comparative test of lidocaine release rate according to the method of adding lidocaine
[0052] 1. Experimental purpose
[0053] The release rates according to the method of adding lidocaine to the hyaluronic acid (hereinafter referred to as "HA") cross-linked composition were compared to confirm the stability.
[0054] 2. Test Substances and Preparation
[0055] 2.1 Test Substances
[0056] Table 1
[0057] category HA concentration (mg / mL) Lidocaine concentration (%) for mixing 24 0.03 for spray 24 10.0
[0058] 2.2 Preparation of test substances
[0059] According to the method of adding lidocaine hydrochloride, test substances were prepared as follows.
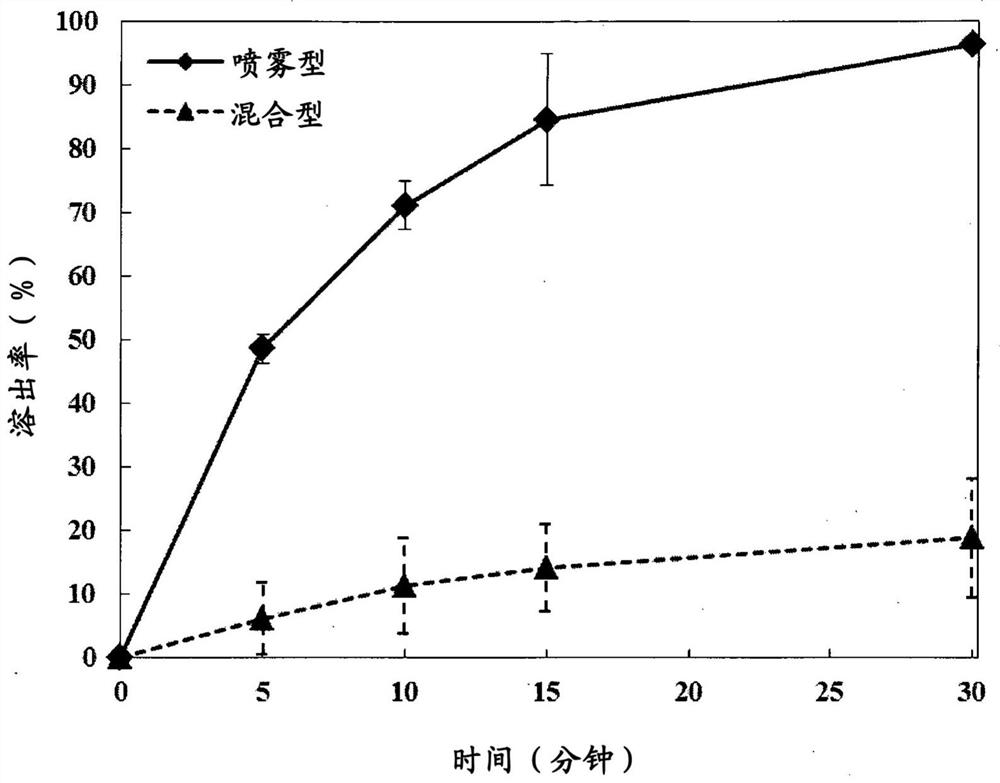
[0060] (1) Gel hybrid type
[0061] After dissolving 0.03 g of lidocaine hydrochloride in 100 mL of PBS, 2.4 g of cross-linked hyaluronic acid was added thereto, and swelling was performed to prepare a hydrogel.
[0062] (2) In-syringe spray type
[0063] ① Put 2.4 ...
Embodiment 2
[0096] Example 2: Comparison of Lidocaine Loading and Dissolution Rates Based on Syringe Material / Syringe Volume
[0097] In order to compare the loading rate and dissolution rate of lidocaine according to syringe material / syringe volume, experiments were carried out under the following conditions to determine the loading rate and dissolution rate of lidocaine.
[0098] 1) Syringe material: Cyclic Olefin Copolymer (COC)
[0099] 2) Syringe volume: 1mL, 3mL, 10mL
[0100]
[0101] -PBS solution, 37°C
[0102] - Lidocaine solution concentration: 10%
[0103] - Method: After spraying 3 times in a syringe, dry in a drying oven at 70°C
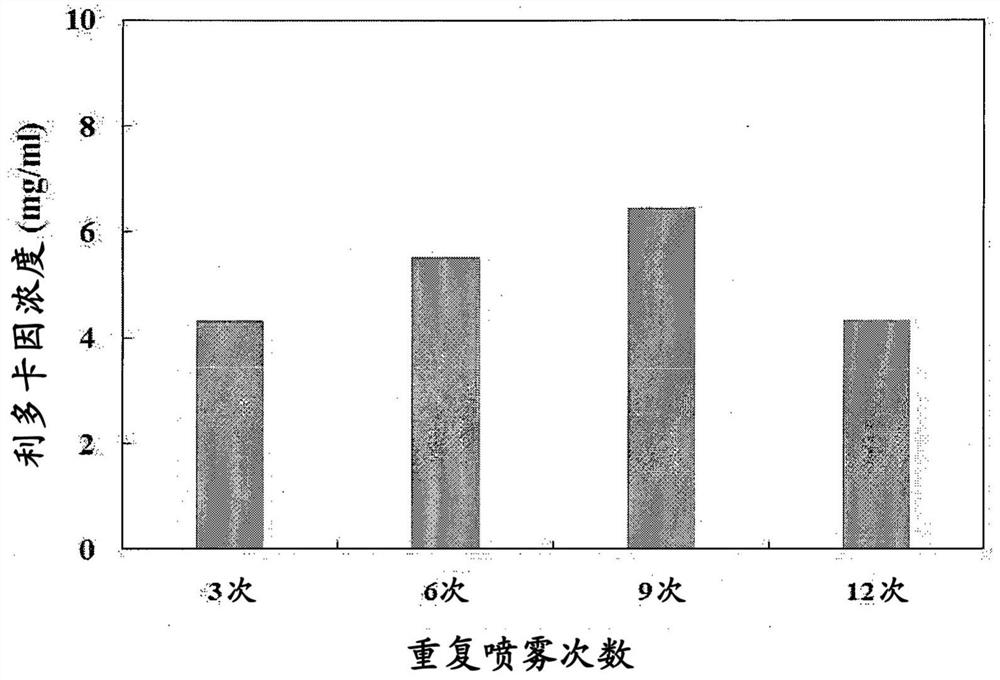
[0104] - Number of spray repetitions: based on 9
[0105] The loading and dissolution rates of lidocaine by syringe volume are shown in Tables 4 and 5 below.
[0106] Table 4 compares the loading rates of lidocaine solutions by syringe volume
[0107] category Lidocaine Concentration (mg / mL) COC 1mL 3.23 COC 3mL 7.27...
Embodiment 3
[0113] Example 3: Confirmation of the effect produced by the composition of the lidocaine spray solution
[0114] In order to confirm whether an injection could be produced according to the hyaluronic acid concentration of the lidocaine spray solution, lidocaine spray solutions were prepared in PBS according to the following concentrations and sprayed, and the results are shown in Table 7 below.
[0115] Table 7
[0116]
[0117] As can be seen from Table 7, when the concentration of hyaluronic acid was 0.1 (w / v)% or more and less than 1.0, it was confirmed that even if the concentration of lidocaine was the same, the injection could be prepared by appropriate spraying.
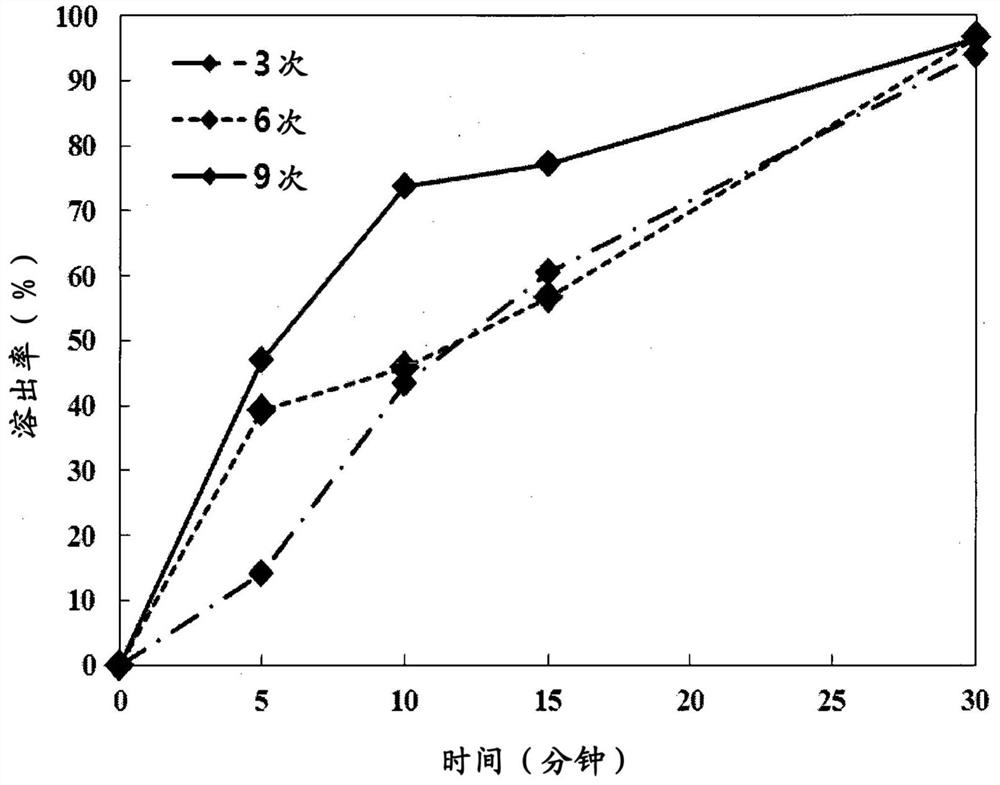
[0118] Then, according to the number of sprays of the lidocaine spray solution containing 0.1 and 0.5 (w / w)% of hyaluronic acid, the loading rate and the dissolution rate showing the desired spray effect of lidocaine were determined. The experimental conditions are as follows. The results are shown in Ta...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com