EGFR L858R neoantigen epitope peptide, and application thereof to tumor treatment
A technology of antigenic epitopes and antigens, applied in the field of molecular immunology, can solve problems such as loss of surgical treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Isolation of peripheral blood mononuclear cells
[0032] Take 20ml of venous blood from HLA-A*1101 healthy donors, dilute the blood sample with an equal volume of sterile PBS, transfer the lymphocyte separation solution (Stemcell, 07861) into a new 50ml centrifuge tube, and ensure that the blood volume ratio is 3 : 4. Carefully add the diluted blood to the surface of the separation liquid, operate as gently as possible, avoid mixing, and clearly see the boundary between the two liquids. Centrifuge at 400g for 30min at room temperature. After centrifugation, suck out the mononuclear cell layer, transfer to a new sterile centrifuge tube, add PBS to wash twice. Cells were resuspended in RPMI-1640 medium and counted.
Embodiment 2
[0033] Example 2 Neoantigen epitope peptide activates specific T lymphocytes
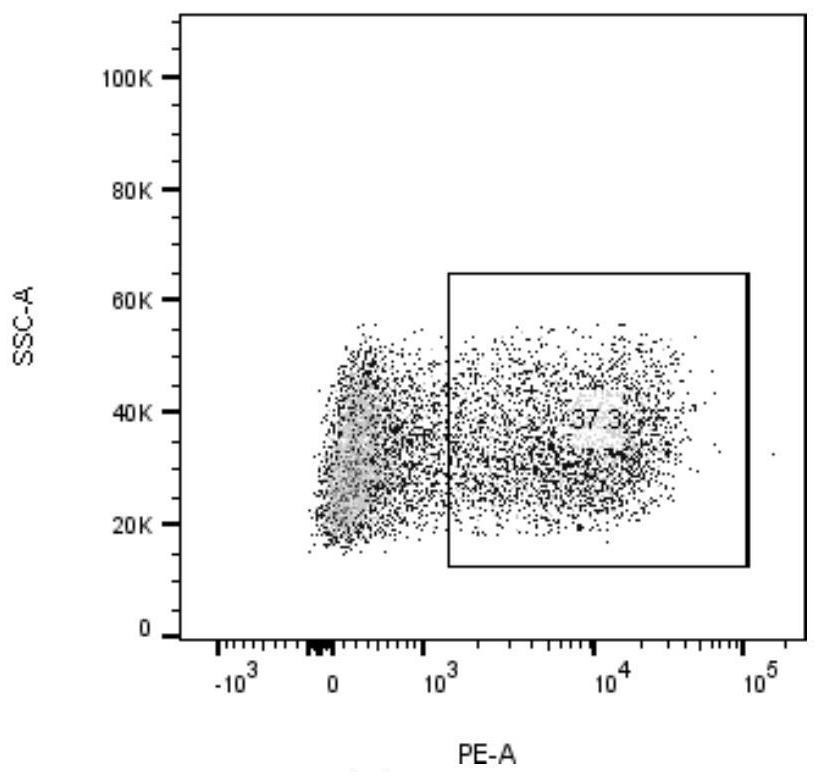
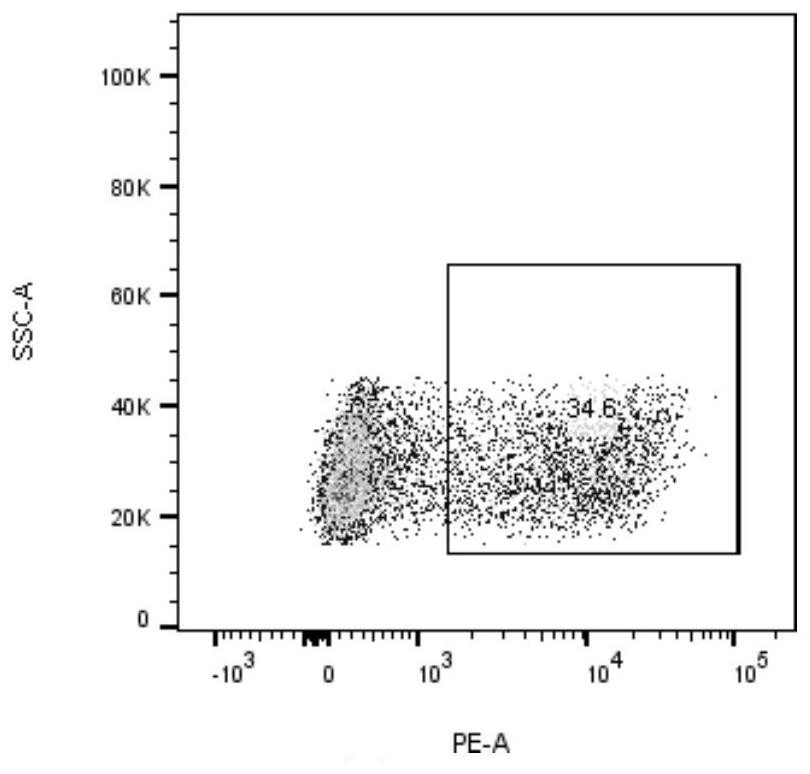
[0034] will be 2.5×10 6 Each cell was suspended in 90% RPMI-1640 + 10% FBS (containing IL-2, 20ng / ml), adding 2.5×10 5 Dynabeads ® Human T-Activator CD3 / CD28 magnetic beads (Gibco, 11131D), inoculated into 6-well plate, 1.25×10 per well 6 cells, two wells were added with neoantigen peptide KITDFGRAK (2.5 μg / ml) and neoantigen peptide VKITDFGRAK (2.5 μg / ml), at 37°C, 5% CO 2 incubate. After 24 hours, resuspend the cells and magnetic beads, place them on the magnetic stand, discard the magnetic beads, collect the supernatant cells and continue to culture for a week, and detect the activated specific cytotoxic T lymphocytes (CTLs) using neoantigen-specific tetramers ),see picture 1, Figure 1A The neoantigen peptide KITDFGRAK specifically caused CTL expression, and the expression rate was 37.3%. Figure 1B The neoantigen peptide VKITDFGRAK can cause specific CTL expression, and the expression ra...
Embodiment 3
[0035] Example 3 Killing of target cells by specific CTLs
[0036] 1. Construct T2 target cells of HLA-A*1101:
[0037]The full-length HLA-A*1101 gene was cloned into the lentiviral expression vector pCDH (purchased from SBI) using restriction endonucleases EcoRI and BamHI to obtain recombinant plasmids to construct the lentiviral expression vector pCDH overexpressing HLA-A*1101 molecules -HLA*1101.
[0038] 293T cells in the logarithmic growth phase (purchased from the Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences) were inoculated into T25 cell culture flasks containing 10% FBS in DMEM medium, and transfected when the cell confluence reached 80-90%. Mix the recombinant plasmid and the mixed packaging vector plasmid pPACKH1 (purchased from SBI), mix well, add 500 μl 1 μg / μl, lipofectamine transfection reagent Lip2000 (Invitrogen, 11668-027), mix immediately, and let stand at room temperature 10-15 minutes. Add the above DNA / liposome complex dropwi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com