Preparation method of 2, 2, 6, 6-tetramethyl-4-aminopiperidine
A technology of tetramethylpiperidone and aminopiperidine is applied in chemical instruments and methods, catalyst activation/preparation, metal/metal oxide/metal hydroxide catalysts, etc. Adverse effects and high production costs, achieving the effect of more application times, low price and less usage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] 329g mass concentration is 30% ammonia water, 3g supported nickel catalyst is added in the hydrogenation tank;
[0057] Add 200g of 2,2,6,6-tetramethylpiperidone into the hydrogenation kettle, and heat up after nitrogen replacement. When the temperature of the hydrogenation kettle rises to 120°C, the hydrogen gas will start the reaction, and the pressure in the hydrogenation kettle will be maintained at 2.0Mpa, the temperature is 120°C until the end of the reaction;
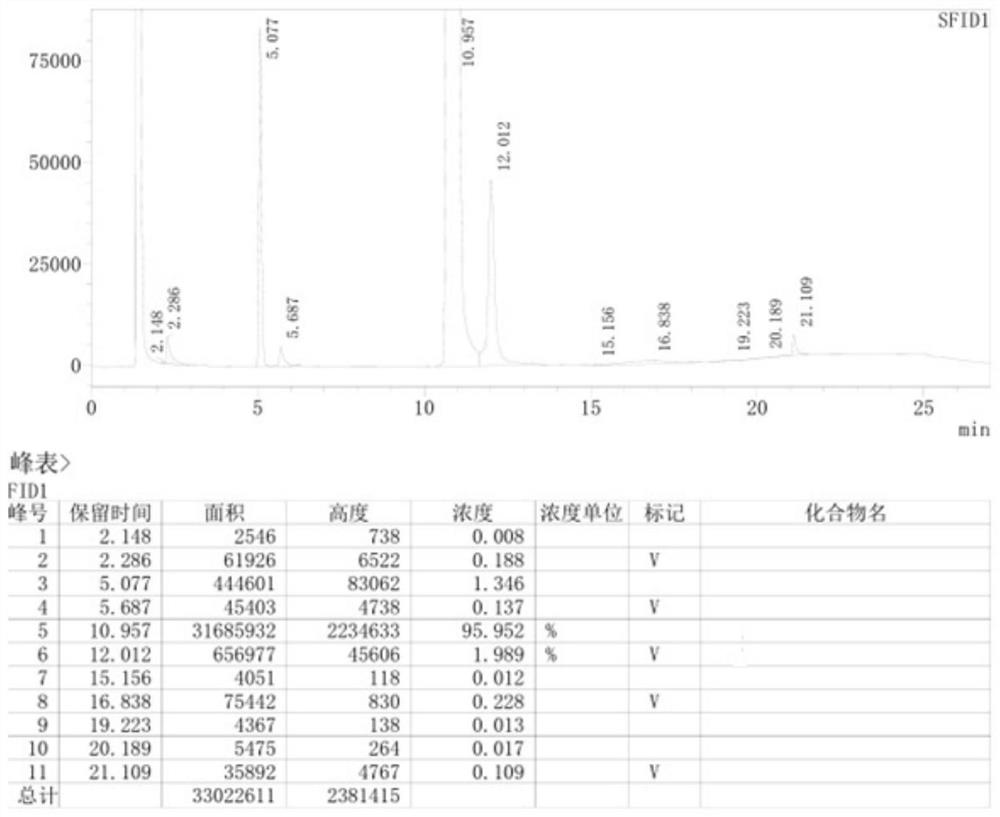
[0058] The GC content of the target product 2,2,6,6-tetramethyl-4-aminopiperidine is 96.54%, and the by-product piperidinol content is 2.06% (see attached figure 1 ).
[0059] Lower the temperature until it falls below 90°C, preferably below 80°C;
[0060] After settling, the reaction system is divided into upper and lower layers, and the catalyst is recovered from the lower layer for mechanical application;
[0061] Filter the supernatant liquid, then rectify, deammoniaize at normal pressure first, rec...
Embodiment 2
[0064] Add 329g30% ammonia water and 2.5g loaded nickel catalyst into the hydrogenation tank
[0065] Add 200g of 2,2,6,6-tetramethylpiperidone into the hydrogenation kettle, heat up after replacement, and when the temperature of the hydrogenation kettle rises to 120°C, the hydrogen gas will start to react, and the pressure in the hydrogenation kettle will be maintained at 2.5 Mpa, temperature is 120 ℃, until reaction finishes;
[0066] The GC content of the target product 2,2,6,6-tetramethyl-4-aminopiperidine is 95.95%, and the by-product piperidinol content is 1.99% ((as attached image 3 shown).
[0067] Lower the temperature until it falls below 90°C, preferably below 80°C;
[0068] After settling, the reaction system is divided into upper and lower layers, and the catalyst is recovered from the lower layer for mechanical application;
[0069] Filter the supernatant liquid, then rectify, deammoniaize at normal pressure first, rectify water at normal pressure, and recove...
Embodiment 3
[0072] It is basically the same as Example 1, except that the temperature in the reaction is 130° C. After the reaction, the GC content of the target product 2,2,6,6-tetramethyl-4-aminopiperidine is 92.39%, while The content of by-product piperidinol is 6.65%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com