Method for diagnosing haemostasis disorders using activated charcoal
An activated carbon and barrier technology, which is applied in the fields of disease diagnosis, biochemical equipment and methods, and microbial determination/examination, and can solve problems such as the relevance of diagnosing hemostatic disorders that have not been considered.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0172] Example 1: Effect of DOAC present in serum samples on aPTT and PT assays for in vitro diagnosis of hemostatic disorders
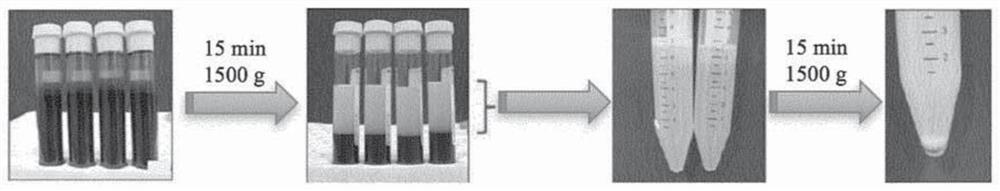
[0173] Standard tests for in vitro diagnosis of hemostatic disorders, such as in hospital laboratories, are usually performed on plasma samples obtained from patients. For the preparation of such plasma samples, the blood components of the patient's blood sample are typically separated by two centrifugation steps, both at 2500g for 15 minutes ( figure 1 ). The first centrifugation step separates the blood into solid matter (eg, red and white blood cells) (ie, the lower phase) and plasma (ie, the upper phase). Subsequently, the plasma is collected and subjected to a second centrifugation step, which pellets residual blood cells and / or platelets. The upper phase (ie platelet poor plasma) obtained by the second centrifugation step can be used for hemostasis tests. However, if the patient has been treated with a direct anticoagulant (eg, DOAC), platel...
Embodiment 2
[0175] Example 2: Methods disclosed herein eliminate the effect of DOACs on aPTT and PT assays for in vitro diagnosis of hemostatic disorders
[0176] Blood samples obtained from subjects were centrifuged at 2500g for 15 minutes to separate the blood into solid matter (eg, red and white blood cells) (ie, lower phase) and plasma (ie, upper phase).
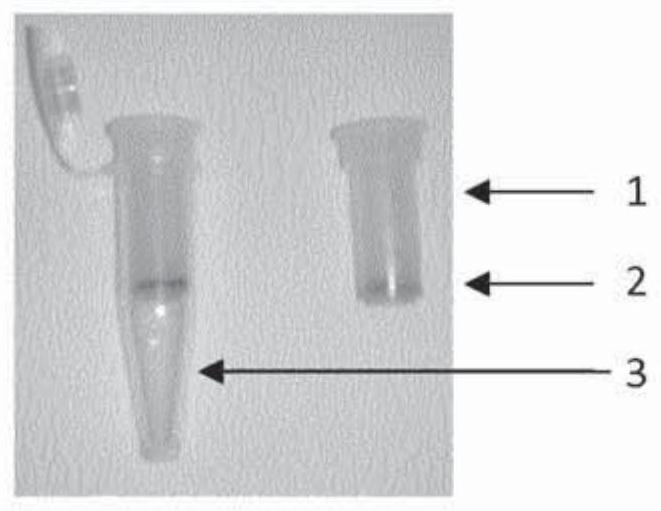
[0177] Plasma obtained from the first (and only) centrifugation step was incubated with charcoal (10 mg / ml plasma) for 5 min, and the plasma was subsequently recovered from the charcoal by passing the plasma through a filter with 0.65 μm pores ( image 3 ). Passing the plasma through the filter was achieved by brief centrifugation. Filters with 0.65 μm pores efficiently remove activated charcoal and residual blood cells and / or platelets with a size greater than 0.65 μm from plasma with minimal interference with blood coagulation tests.
[0178] Thus, it appears that recovery of plasma from activated charcoal by passing the plasma ...
Embodiment 3
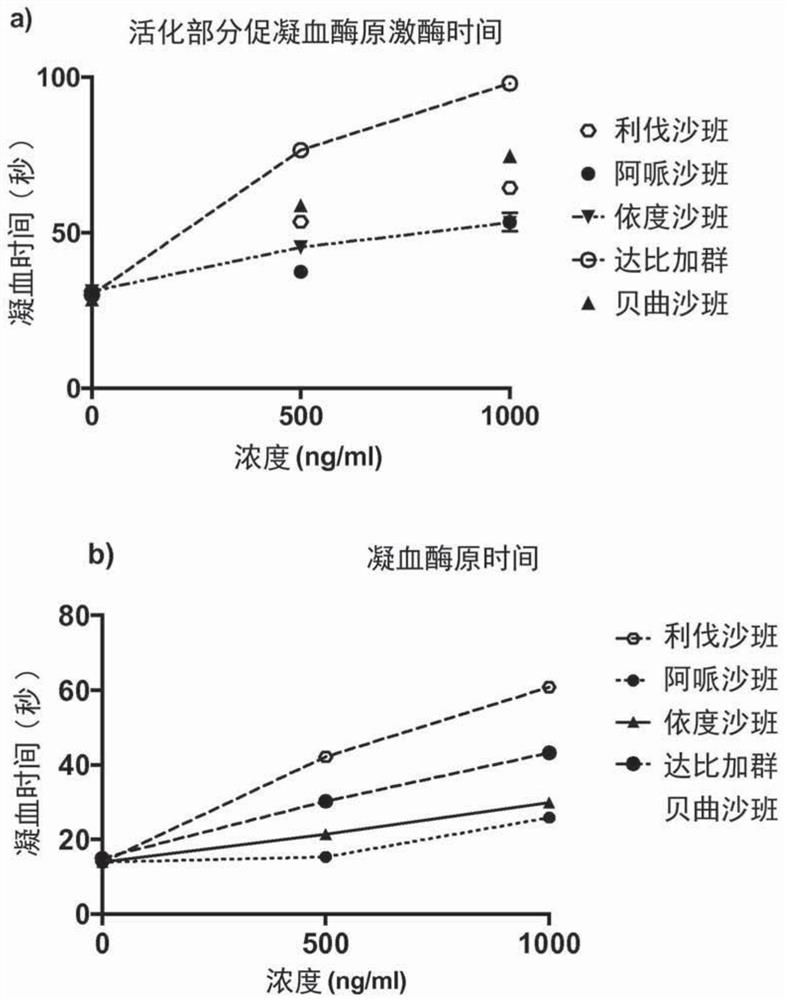
[0180] Example 3: Effect of Activated Carbon Concentration on Elimination of DOAC Effects on aPTT and PT Determinations
[0181] Plasma was obtained from blood samples obtained from subjects by one centrifugation as described in Example 2. Plasma samples were incubated with different concentrations of activated charcoal (i.e. 5 mg / ml, 10 mg / ml or 15 mg / ml) for 5 min and then recovered from the activated charcoal by passing the plasma through a filter with 0.65 μm pores and activated charcoal by brief centrifugation.
[0182] Filtered plasma samples were used for aPTT and PT determinations. The results showed that even at high concentrations of rivaroxaban (e.g., 1000 ng / ml), the effect of rivaroxaban on the determination of aPTT and PT was completely abolished ( Figure 5 a and 5b). Also, 5 mg of activated charcoal per ml is enough to achieve this effect.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com