Styrene cyclooxygenase derived from rhizobium, and function of styrene cyclooxygenase
A cyclooxygenase, styrene technology, applied in the direction of oxidoreductase, enzymes, microorganism-based methods, etc., to achieve the effect of excellent enantioselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
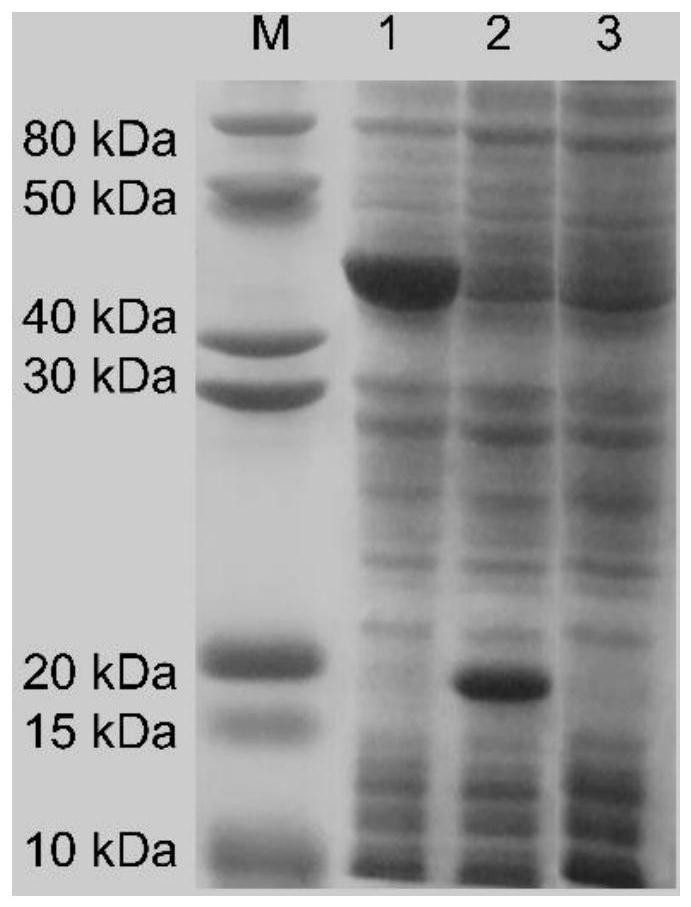
[0021] Example 1 BrSMO heterologous expression
[0022] After the plasmid pET-BrSMO was transformed into Escherichia coli BL21(DE3) competent, a single clone was picked and inoculated in LB medium containing kanamycin (50 mg / L), and cultivated overnight at 37°C and 180 rpm for use as seed liquid. Transfer the seed solution to 200mL TB medium containing kanamycin (50mg / L) with 1% inoculation rate, and cultivate it at 37°C and 180rpm for 3h, when the OD 600 After reaching 0.8, add IPTG (final concentration 0.05mM) and induce at 20°C for 20 hours. Then centrifuge at 8000 rpm at 4° C. for 10 minutes to obtain the bacterial cells, and then resuspend and wash twice with 0.9% NaCl solution to obtain wet bacterial cells. The wet bacteria are used as a biocatalyst for subsequent biocatalysis.
[0023] The obtained wet bacteria were resuspended in potassium phosphate buffer (0.1M, pH 7.0), and the cells were crushed with a high-pressure homogenizer to obtain a cell disruption liquid,...
Embodiment 2
[0024] Example 2 Optimization of styrene cyclooxygenase BrSMO whole cell biocatalysis conditions
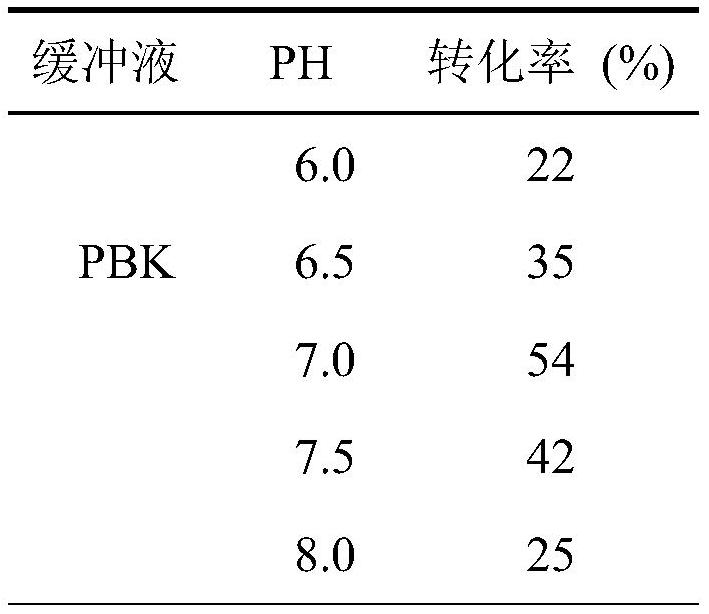
[0025] 2.1 Optimum reaction pH value optimization
[0026] The reaction system was 5 mL, including 0.5 g of E. coli (pET-BrSMO) wet cells, 0.1 M potassium phosphate buffer PBK (6.0, 6.5, 7.0, 7.5, 8.0) of different pH, cyclohexane (10%) and Styrene (8mM), shaking reaction at 30°C and 200rpm for 2h. After the reaction was completed, an equal volume of ethyl acetate was used to stop the reaction and extracted, and an appropriate amount of anhydrous sodium sulfate was added to dry it, and the mixture was detected and analyzed by GC. The instrument used is Agilent Technologies 7890B GC gas chromatograph, the chiral column is Cyclodex-B column (30m×0.25mm×0.25μm, USA), the injector temperature, detector sample temperature and column temperature are 260°C and 280°C respectively and 100°C. The results are shown in Table 2. The epoxy production rate is the highest in the 0.1M potassiu...
Embodiment 3
[0035] Embodiment 3 Styrene cyclooxygenase BrSMO carries out biotransformation to different substrates
[0036] The reaction system was 5mL, including 0.1M potassium phosphate buffer (pH 7.0), 0.5g E.coli (pET-BrSMO) wet cells, 100μL isopropanol, 5mM substrate (see Table 4, Table 5), at 30 ℃, 200rpm shaking reaction for 2 hours, stop the reaction with ethyl acetate and extract, add anhydrous sodium sulfate for drying, rotary evaporation to remove the solvent, GC and HPLC detection and analysis, see Table 6 and Table 7. Instrument used for GC detection: Agilent Technologies 7890B GC system-FID detector. Instrument used for HPLC detection: Shimadzu Prominence LC-20AD system-PDA detector.
[0037] It can be seen from Table 4 that the styrene cyclooxygenase BrSMO disclosed in the present invention converts 8 kinds of styrene substrates into corresponding epoxy products, all of which have S-type selectivity. Except for 2a (97% ee) and 5a (95% ee), it showed excellent enantioselec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com