Synthesis method of pyrimidine heterocyclic ring-containing antitumor medicine molecule AZD6738
A technology of AZD6738 and compound, which is applied in the field of synthesis of pyrimidine-containing heterocyclic anti-tumor drug molecule AZD6738, can solve the problems of expensive raw materials and reactors, large investment in process production, etc., and achieve the effect of convenient purification and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Embodiment 1 AZD6738 batch synthesis:
[0084] (1) The first step: the synthesis of compound 3:
[0085]
[0086]Compound 2, methyl 2,6-dichloropyrimidine-4-carboxylate (1000g) was dissolved in dichloromethane (5000 ml), compound 1 (480g) and TEA (900g) were added, the reaction solution was stirred at room temperature for 16h, LC-MS Tracking showed that the reaction was complete, and 1200mL of water was added to the reaction solution, separated and extracted, the aqueous phase was extracted with dichloromethane (4000mL*2), and the organic phases were combined. The organic phase was dried over anhydrous sodium sulfate, filtered, and concentrated by rotary evaporation in vacuo to obtain crude compound 3. Afterwards, it was purified by beating with a volume ratio of MTBE:EA=4:1 to obtain a light yellow solid powder (920 g, yield: 70%).
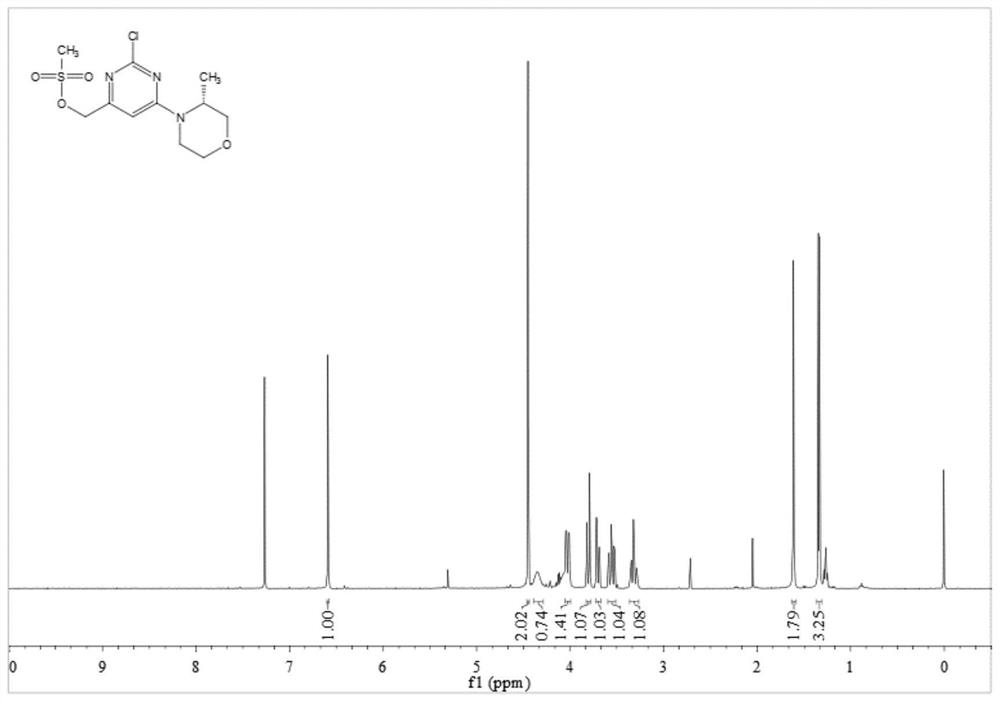
[0087] 1 H NMR (400 MHz, CDCl 3 ) δ 7.17 (s, 1H), 4.39 (s, 1H), 4.41 (d, J =13.2 Hz, 1H), 4.26-4.07 (m, 1H), 3.99 (s, 3H), 3.82 ...
Embodiment 2
[0120] Embodiment two: the fluid chemical synthesis method of AZD6738:
[0121] (1) In the first step, compound 1 ((R)-2-methylmorpholine, 48g) and triethylamine (90g) were dissolved in dichloromethane solution to form solution A of 250mL, and compound 2 ( 100g) was also dissolved in dichloromethane, and made into 250mL solution B. According to the set flow rate of 2.77mL / min, the two kinds of A and B dichloromethane solutions were respectively pumped into a T-shaped mixer. Then enter a coil reactor with a set temperature, the retention volume of the coil reactor is 250mL, the temperature is 36°C, and the retention time of the set material in the reactor is 45min. Inject water (300mL) through another pump at a rate of 5mL / min to quench the reaction solution coming out of the coil reactor, and then enter the membrane separator (Zaiput brand) to achieve liquid-liquid separation, and the water phase continues to enter the lower The first-stage liquid-liquid separator was pumped ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com