Preparation technology of roflumilast film-coated tablets
A technology of roflumilast and film coating, which is applied in the field of tablet preparation, can solve the problem of difficulty in realizing tablet production quickly and efficiently, and achieves good batch-to-batch reproducibility, less fine powder, and smooth plain tablets. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The preparation of the first batch of samples (batch number 120301) and the study of the main process parameters were carried out in small scale scale-up.
[0039] Equipment: SHK-6 laboratory fast wet granulator, YK60 swing granulator, DHG-9203A electric blast drying oven, ZP5 rotary tablet press, GBB-38 laboratory high-efficiency coating machine.
[0040] (1) Screening and mixing of raw and auxiliary materials: take roflumilast raw materials and micronize them (particle size range: D(90)=13.498, D(50)=5.398, D(10)=1.086), and set aside; lactose, starch, Magnesium stearate is passed through an 80-mesh sieve respectively, and is set aside. Mix the prescription amount of roflumilast with about 13% of the prescription amount of starch in equal increments; set aside (the weight after mixing accounts for about 3.1% of the total prescription amount). The roflumilast starch mixture was mixed with the prescription amount of lactose and remaining starch in a laboratory rapid we...
Embodiment 2
[0058] The preparation of the second batch of samples (batch number 120302) and the study of the main process parameters were carried out in small scale scale-up.
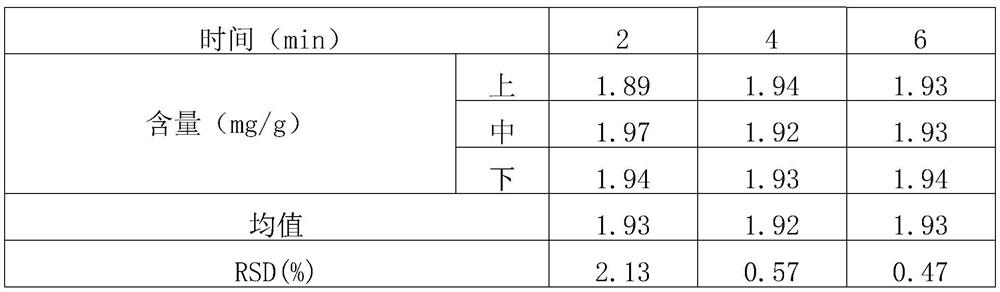
[0059] (1) Screening and mixing of raw and auxiliary materials: micronize the raw materials of roflumilast (particle size range: D(90)=13.498, D(50)=5.398, D(10)=1.086), and set aside; lactose, starch, Magnesium stearate is passed through an 80-mesh sieve respectively, and is set aside. Mix the prescription amount of roflumilast with about 13% of the prescription amount of starch in equal increments; set aside (the weight after mixing accounts for about 3.1% of the total prescription amount). The roflumilast starch mixture was mixed with the prescribed amount of lactose and remaining starch in a laboratory rapid wet granulator (stirrer speed 400 rpm, cutter speed 600 rpm, 4 minutes). Samples were taken at the upper, middle and lower positions to determine the content, and the RSD was calculated. The results are sh...
Embodiment 3
[0074] The preparation of the third batch of samples (batch number 120303) and the study of the main process parameters were carried out in small scale scale-up.
[0075] (1) Screening and mixing of raw and auxiliary materials: micronize the raw materials of roflumilast (particle size range: D(90)=13.498, D(50)=5.398, D(10)=1.086), and set aside; lactose, starch, Magnesium stearate, pass through 80 mesh sieves respectively, and set aside. Mix the prescription amount of roflumilast with about 13% of the prescription amount of starch in equal increments; set aside (the weight after mixing accounts for about 3.1% of the total prescription amount). The roflumilast starch mixture was mixed with the prescribed amount of lactose and remaining starch in a laboratory rapid wet granulator (stirrer speed 400 rpm, cutter speed 600 rpm, 4 minutes). Samples were taken at the upper, middle and lower positions to determine the content, and the RSD was calculated. The results are shown in Tab...
PUM
| Property | Measurement | Unit |
|---|---|---|
| angle of repose | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com