Nitrogen-doped carbon layer coated cobalt manganese carbide composite material and application thereof
A cobalt-manganese carbide composite material and nitrogen-doped carbon technology, applied in the field of nanomaterials and photocatalysis, can solve the problems of limited reserves, high cost, unfavorable large-scale application, etc., and achieve improved hydrogen production activity, simple synthesis, and novel structure Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The present embodiment nitrogen-doped carbon-coated nano-Mn 2 co 2 The preparation method of C, concrete preparation steps are as follows:
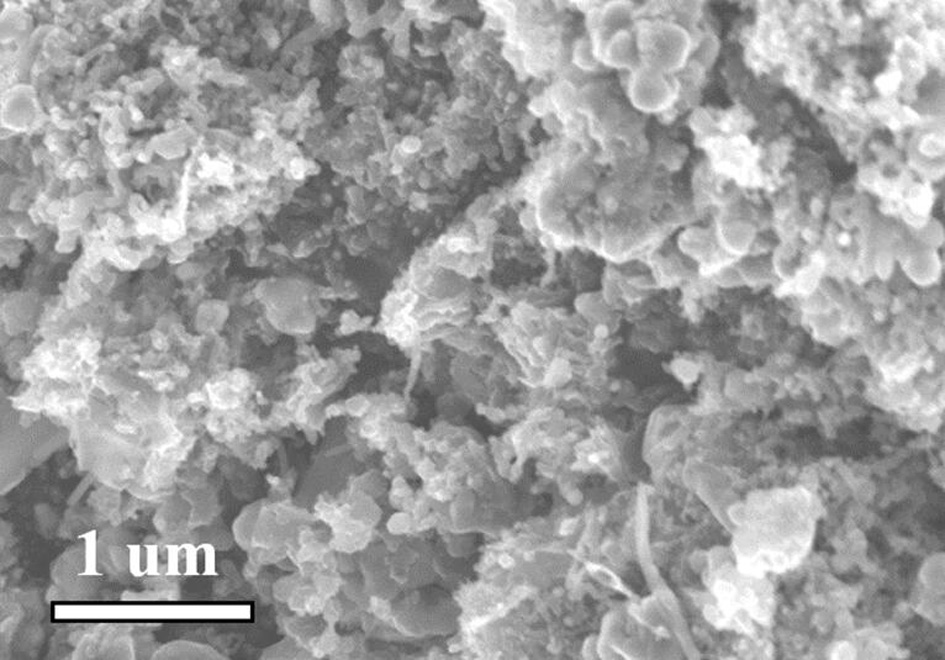
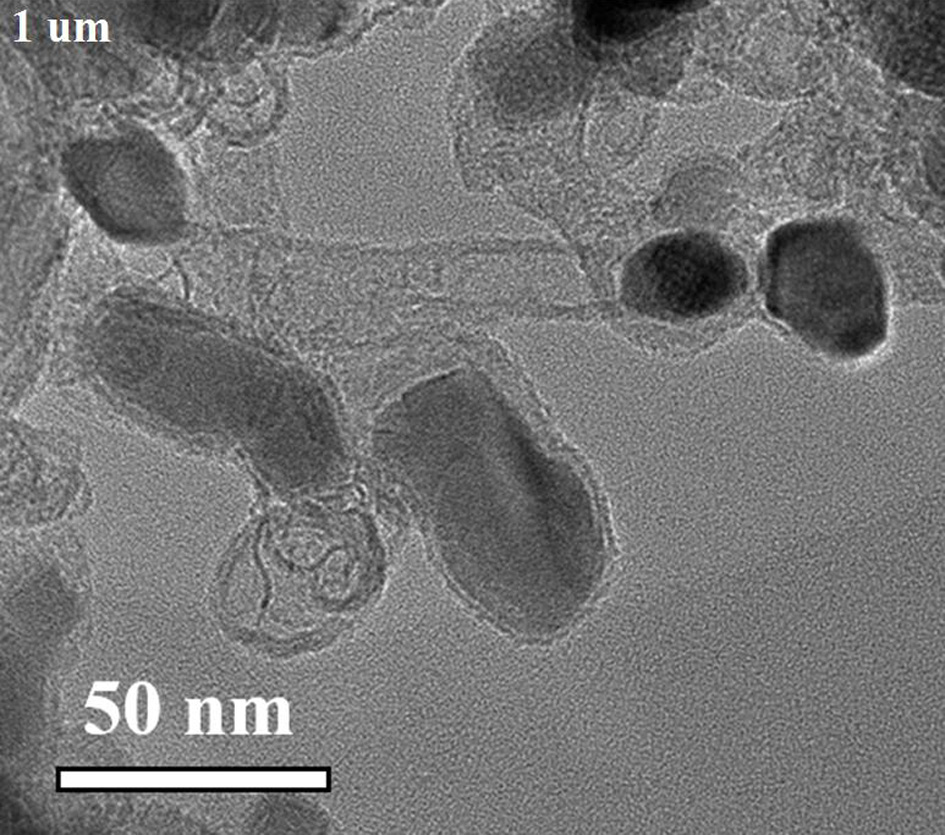
[0035] Will Co(NO 3 ) 2 ·6H 2 O (1.0 mmol, 291.0 mg) and Mn(NO 3 ) 2 4H 2 O (5.0 mmol, 1255.1 mg) was dissolved in 100 mL deionized water and stirred to dissolve. Will K 3 Co(CN) 6 (4.0 mmol, 1329.3 mg) was added to the above solution, stirred to dissolve, and then left to age for 24 h. The precipitate was separated by centrifugation and dried overnight at 80°C to obtain cobalt manganese Prussian blue. Grind the dried samples evenly and place them in a tube furnace under N 2 Under the protection of the atmosphere, at 5°C·min -1 The heating rate was raised to 800°C, and the target product nitrogen-doped carbon-coated nano-Mn was obtained after pyrolysis for 2 hours. 2 co 2 C (Mn 2 co 2 C@C).
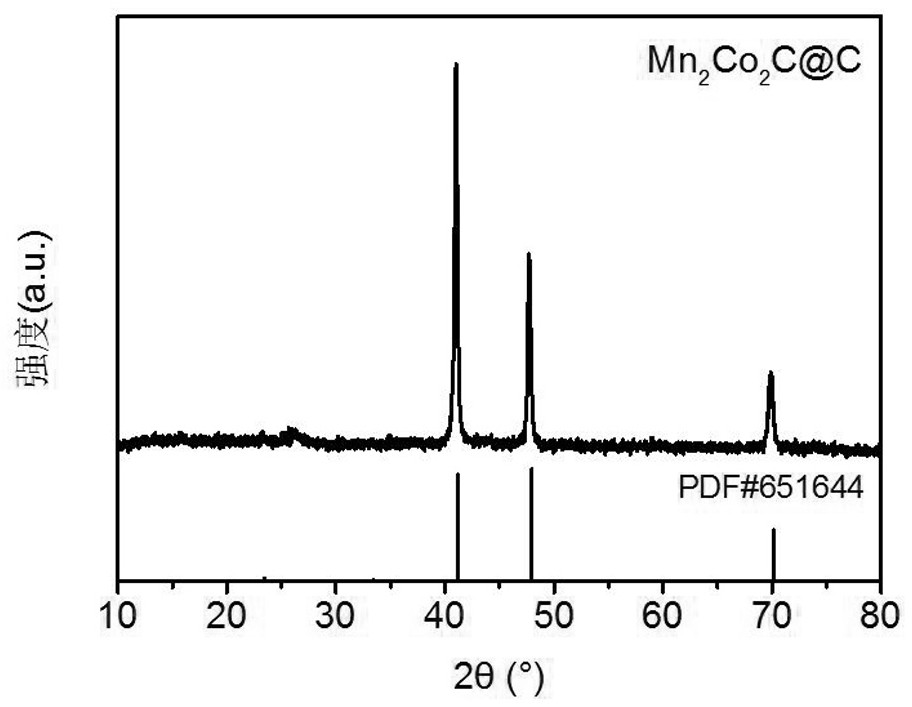
[0036] Mn obtained in this example 2 co 2 The XRD pattern of C@C is as follows figure 1 As shown, Mn 2 co 2 The XRD pa...
Embodiment 2
[0042] The present embodiment nitrogen-doped carbon-coated nano-Mn 2 co 2 The preparation method of C, the specific preparation steps are as follows: Co(NO 3 ) 2 ·6H 2 O (1.0 mmol, 291.0 mg) and Mn(NO 3 ) 2 4H 2 O (5.0 mmol, 1255.1 mg) was dissolved in 100 mL deionized water and stirred to dissolve. Will K 3 Co(CN) 6 (4.0 mmol, 1329.3 mg) was added to the above solution, stirred to dissolve, and then left to age for 24 h. The precipitate was separated by centrifugation and dried overnight at 80°C to obtain cobalt manganese Prussian blue. Grind the dried samples evenly and place them in a tube furnace under N 2 Under the protection of the atmosphere, at 5°C·min -1 The heating rate was raised to 700°C, and the target product nitrogen-doped carbon-coated nano-Mn was obtained after the pyrolysis reaction was completed for 4 hours. 2 co 2 C (Mn 2 co 2 C@C).
Embodiment 3
[0044] The present embodiment nitrogen-doped carbon-coated nano-Mn 2 co 2 The preparation method of C, the specific preparation steps are as follows: Co(NO 3 ) 2 ·6H 2 O (1.0 mmol, 291.0 mg) and Mn(NO 3 ) 2 4H 2O (5.0 mmol, 1255.1 mg) was dissolved in 100 mL deionized water and stirred to dissolve. Will K 3 Co(CN) 6 (4.0 mmol, 1329.3 mg) was added to the above solution, stirred to dissolve, and then left to age for 24 h. The precipitate was separated by centrifugation and dried overnight at 80°C to obtain cobalt manganese Prussian blue. Grind the dried samples evenly and place them in a tube furnace under N 2 Under the protection of the atmosphere, at 5°C·min -1 The heating rate is raised to 900 ° C, and after the pyrolysis reaction is completed for 2 hours, the target product nitrogen-doped carbon-coated nano-Mn 2 co 2 C (Mn 2 co 2 C@C).
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com