High performance liquid chromatography analysis method for dihydralazine sulfate related substances
A technology of dihydralazine sulfate and high performance liquid chromatography is applied in the field of ultra-high performance liquid chromatography analysis of dihydralazine sulfate and related substances of its tablets, and can solve the problem that it is difficult to effectively control the dihydralazine sulfate and its tablets. drug product quality, safety risks and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: detection instrument and chromatographic conditions:
[0029] High performance liquid chromatography: Waters UPLC AcQuity H-Class
[0030] Chromatographic column: the reverse phase C18 column is selected from Infinity Lab Poroshell 120 SB-C18, the specification is 4.6*150mm, 2.7Micron.
[0031] Mobile phase A: Take 2.0g of anhydrous potassium dihydrogen phosphate, add 1000ml of water to dissolve, adjust the pH to 1.8 with phosphoric acid;
[0032] Mobile phase B: Methanol;
[0033] Detection wavelength: 228nm;
[0034] Flow rate: 0.35ml / min
[0035] Column temperature: 5°C
[0036] Gradient elution program:
[0037] time (min) Mobile phase A(%) Mobile phase B(%) 0 98 2 17 60 40 23 0 100 25 98 2
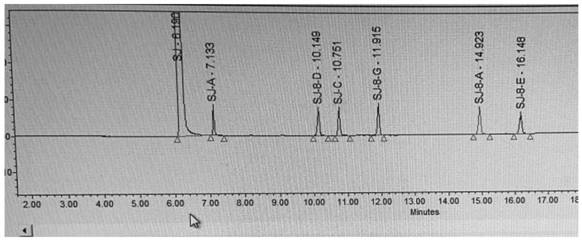
[0038] Preparation of the reference solution: Take impurities A, B, C, D, E, F, about 5 mg each, in a 50ml volumetric flask, add diluent to dissolve and dilute to the mark, shake well, as the stock solution of the re...
Embodiment 2
[0048] Embodiment 2: detection instrument and chromatographic conditions:
[0049] Ultra-Performance Liquid Chromatography: Waters UPLC AcQuity H-Class
[0050]Chromatographic column: the reversed-phase C18 column is selected from Infinity Lab Poroshell 120 SB-C18, the specification is 4.6*150mm, 2.7Micron.
[0051] Mobile phase A: Take 2.0g of anhydrous potassium dihydrogen phosphate, add 1000ml of water to dissolve, and adjust the pH to 2.0 with phosphoric acid;
[0052] Mobile phase B: Methanol;
[0053] Detection wavelength: 230nm;
[0054] Flow rate: 0.40ml / min
[0055] Column temperature: 15°C
[0056] The gradient elution procedure is the same as in Example 1.
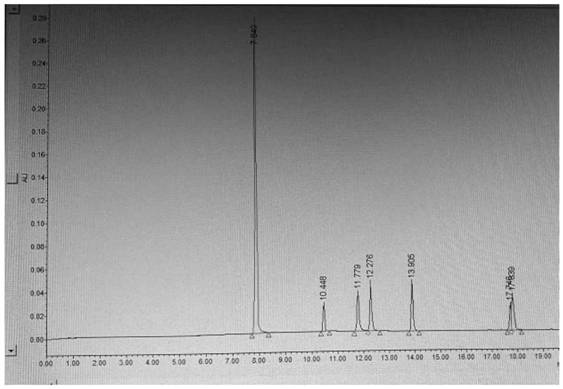
[0057] For the results, see the attached image 3 .
Embodiment 3
[0058] Embodiment 3: detection instrument and chromatographic conditions:
[0059] Ultra-Performance Liquid Chromatography: Waters UPLC AcQuity H-Class
[0060] Chromatographic column: the reverse phase C18 column is selected from Infinity Lab Poroshell 120 SB-C18, the specification is 4.6*150mm, 2.7Micron.
[0061] Mobile phase A: Take 2.0g of anhydrous potassium dihydrogen phosphate, add 1000ml of water to dissolve, and adjust the pH to 2.2 with phosphoric acid;
[0062] Mobile phase B: Methanol;
[0063] Detection wavelength: 232nm;
[0064] Flow rate: 0.45ml / min
[0065] Column temperature: 25°C
[0066] The gradient elution procedure is the same as in Example 1.
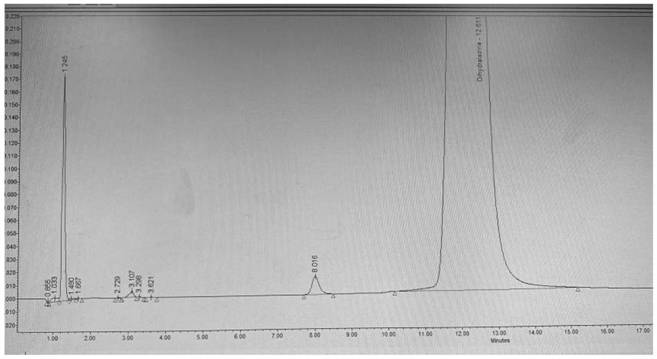
[0067] For the results, see the attached Figure 4 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com