Coding gene of alpha-L-rhamnosidase mutant and expression vector of coding gene
A technology of rhamnosidase and coding gene, which is applied in the fields of glycosylase, genetic engineering, plant gene improvement, etc., to achieve the effect of improving enzyme activity and heat resistance stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Embodiment 1: Obtaining the coding gene of sequence mutation α-L-rhamnosidase mutant
[0014] In order to improve the temperature stability of wild-type α-L-rhamnosidase, the applicant conducted point mutation screening on the wild-type α-L-rhamnosidase gene (GenBank: AF284762.1), and finally screened for temperature stability Increased sequence mutation gene. The mutated nucleotide sequence is shown in SEQ ID NO:1. The wild-type α-L-rhamnosidase gene and the sequence of SEQ ID NO: 1 with restriction sites (EcoR I and Not I) were synthesized by chemical synthesis method.
Embodiment 2
[0015] Embodiment 2: Expression of sequence mutation α-L-rhamnosidase mutant gene
[0016] The wild-type α-L-rhamnosidase gene, the sequence mutation α-L-rhamnosidase mutant gene and the pPIC9K vector were cut with EcoR I and Not I respectively, and the wild-type α-L-rhamnosidase Gene and sequence mutation The α-L-rhamnosidase mutant gene was connected to the EcoR I and Not I sites of the pPIC9K vector to obtain recombinant vectors pPIC9K-R (containing the wild-type α-L-rhamnosidase gene) and pPIC9K -RM (alpha-L-rhamnosidase mutant gene containing sequence mutation). Transfer the recombinant vector pPIC9K-R into Pichia pastoris GS115 to obtain recombinant Pichia RhaO containing the wild-type α-L-rhamnosidase gene, and transfer the recombinant vector pPIC9K-RM into Pichia GS115 to obtain sequence mutation α-L - Recombinant Pichia RhaR of the rhamnosidase mutant gene.
[0017] Recombinant Pichia pastoris RhaO and recombinant Pichia pastoris RhaR were inoculated into 50mL liqui...
Embodiment 3
[0019] Example 3: Temperature stability analysis of α-L-rhamnosidase mutants
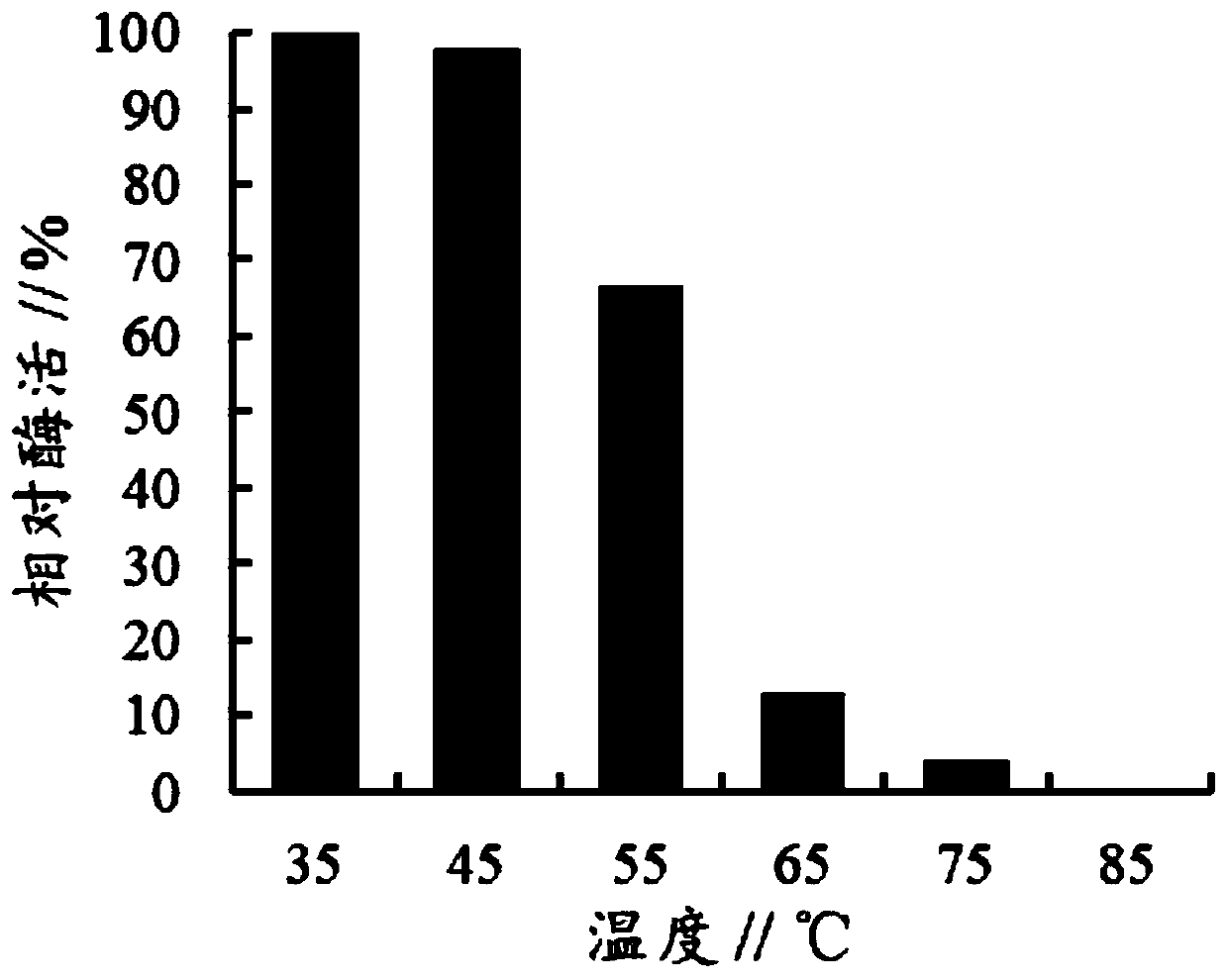
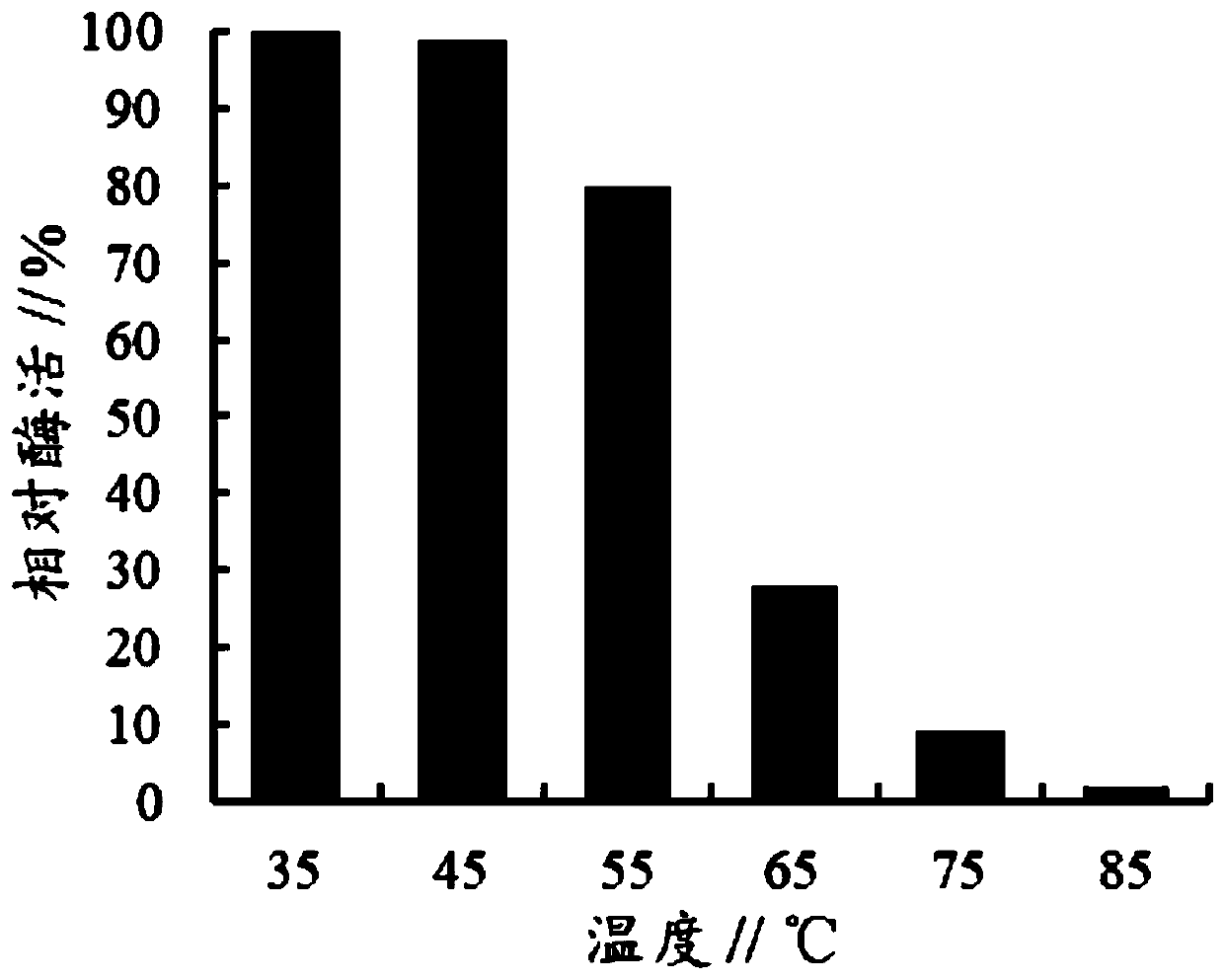
[0020] The enzyme-producing supernatants of the recombinant Pichia pastoris strain containing the original α-L-rhamnosidase gene and the recombinant Pichia pastoris strain containing the mutant α-L-rhamnosidase mutant gene in Example 3 were respectively Store and incubate at 35°C, 45°C, 55°C, 65°C, 75°C and 85°C for 10 minutes, take samples to measure α-L-rhamnosidase activity, take the initial α-L-rhamnosidase activity as 100 %Calculation of relative enzyme activity, the result shows ( figure 1 and figure 2 ) The heat-resistant stability of the α-L-rhamnosidase mutant of the present invention is significantly improved, and after being incubated at 65° C. for 10 min, it can still maintain 28% of the enzyme activity, while the wild-type α-L-rhamnosidase Only 13% of the enzyme activity remained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com