A kind of pharmaceutical composition comprising sunitinib and its preparation and application
A technology of sunitinib and its composition, which is applied in the field of tumor treatment, can solve the problems of reducing sunitinib drug resistance, severe drug resistance, and reducing the therapeutic effect, so as to improve drug safety, significantly kill efficiency, The effect of broad market prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1 Cell Proliferation Inhibition Experiment in Vitro
[0041] (1) Take 786-O cells in the logarithmic growth phase and plant them in a 96-well plate at a certain density (1500-2000 / well);
[0042] (2) Discard the old medium after 24 hours, divide the cells into 5 groups of 1-5 for use, add medium containing different drug concentrations, set 3 duplicate wells for each group, and add sunitinib alone to group 1 Sunitinib and 10 μM apatinib were added to group 2, sunitinib and 10 μM apatinib were added to group 3, DMSO was added to group 4, and group 5 was used as a blank group;
[0043] (3) After culturing for 48 hours, discard the drug solution, add medium containing 5% CCK-8, incubate at 37°C for 3 hours, and then use a microplate reader to detect the OD value at 450nm.
[0044] Based on the DMSO group, the cell survival rate = (OD value of the drug-dosed group - OD value of the blank group) / (OD value of the DMSO group - OD value of the blank group) × 100%, co...
Embodiment 2
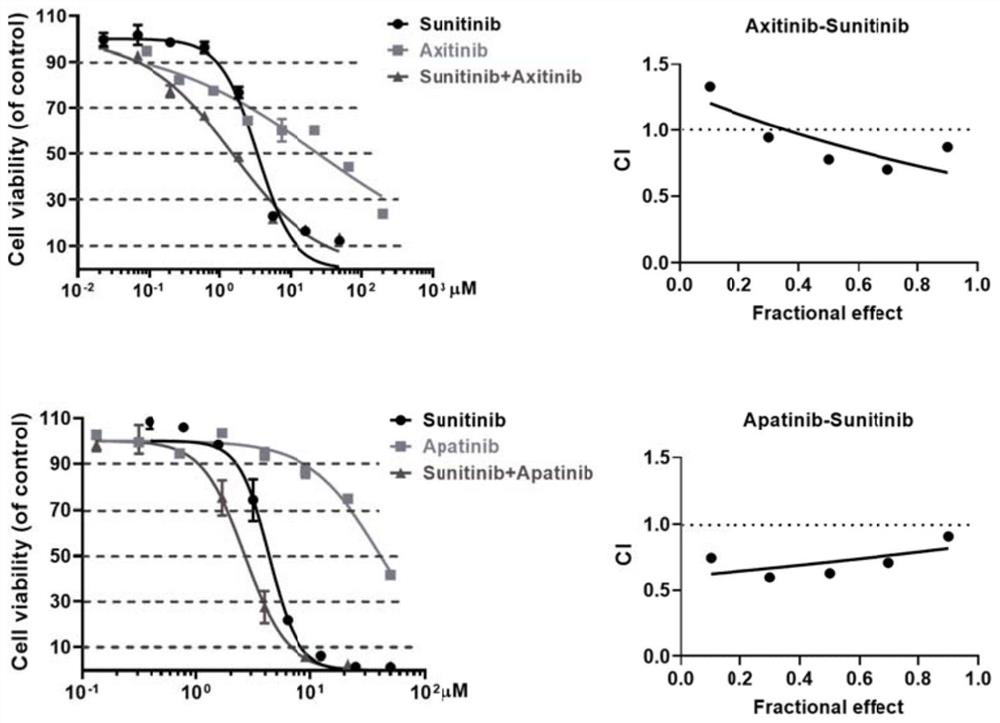
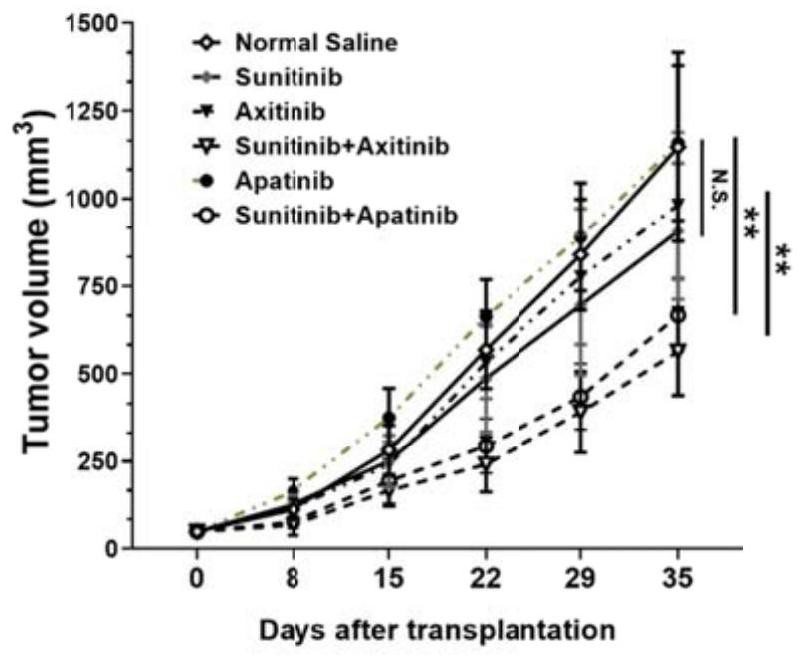
[0048] Example 2 In vitro anti-tumor synergy experiment
[0049] In this experiment, on the basis of the in vitro cell proliferation inhibition experiment stated in Example 1, by measuring the 786-O cells at 10%, 30%, 50%, 70% and 90% inhibition rate, single drug (Apa Apatinib, Axitinib, or Sunitinib) and the drug concentration required for combination therapy (Apatinib + Sunitinib or Axitinib + Sunitinib), so as to calculate the combination index using the formula (Combination Index, CI), make a Fa-CI curve: where the CI calculation formula is as follows:
[0050] CI = D 1x / D 1 + D 2x / D 2 +( D 1x · D 2x ) / ( D 1 · D 2 ).
[0051] D 1x : The concentration of the first drug when the combination group reaches a certain cell inhibition rate; D 2x : The concentration of the second drug when the combination group reaches a certain cell inhibition rate; D 1 : The concentration of the first drug when the single-drug group reaches a certain cell inhibition ...
Embodiment 3
[0056] Example 3 In vitro anti-tumor synergistic compatibility experiment
[0057] (1) The 786-O, A498 and Caki-1 cells in the logarithmic growth phase were planted in a 96-well plate at a certain density (1500-2000 / well);
[0058] (2) Discard the old medium after 24 hours, add drugs containing different compatible forms to continue the culture, and set 3 duplicate wells for each group;
[0059] (3) After culturing for 48 hours, discard the cell culture medium, add a medium containing 5% CCK-8, incubate at 37°C for 3 hours, and then use a microplate reader to detect the OD value at 450nm.
[0060] Based on the DMSO group, the corresponding synergistic effect evaluation was carried out on the inhibition rate, and the combined drug effect was analyzed by King's formula (Q value):
[0061] Q=E (A+B) / (E A +E B -E A ×E B ), that is, E (A+B) : Inhibition rate of combination drug, E A : Inhibition rate of drug A alone, E B : Inhibition rate of drug B alone. When Q>1.15, it...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com