4-aminopyridazinone compound and preparation method thereof
A technology of aminopyridazinone and compounds, which is applied in the field of organic chemical synthesis, can solve problems such as harsh reaction conditions, potential safety hazards, and equipment corrosion, and achieve the effects of mild reaction conditions, avoiding high-temperature reflux, and high yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040]
[0041] Synthesis of compound 4a: Take a dry hard reaction tube, add α-bromohydrazone 1a (36.6 mg, 0.1 mmol) into the reaction tube, add azlactone 2a (35.6 mg, 0.15 mmol), and then add anhydrous carbonic acid Sodium (10.6 mg, 0.1 mmol), and finally anhydrous toluene (2 mL) was added to replace the nitrogen, and the reaction was carried out at room temperature for 24 h under the protection of nitrogen. After the reaction was completed, the solvent was concentrated under reduced pressure, separated and purified by column chromatography (volume ratio: petroleum ether / ethyl acetate=10 / 1-3 / 1) to obtain compound 4a as a white solid, the purity of which was detected by HPLC was 99%, and the yield was 98%.
[0042] Scale-up reaction: Scale up the reaction until the feed amount of α-halohydrazone 1a is 1.0 g. After the reaction is completed, concentrate under reduced pressure and recover the reaction solvent toluene. After concentrating to dryness, add CH to the system 2 C...
Embodiment 2
[0044]
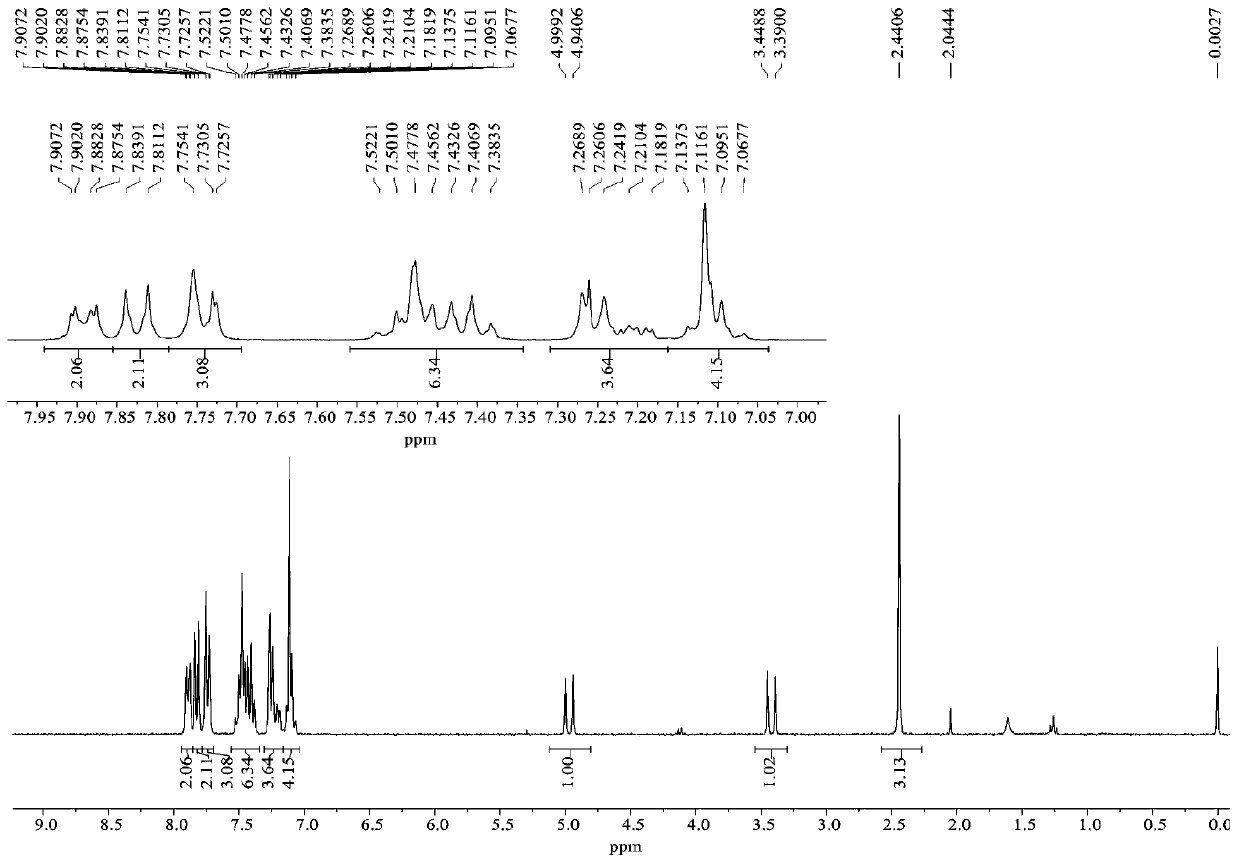
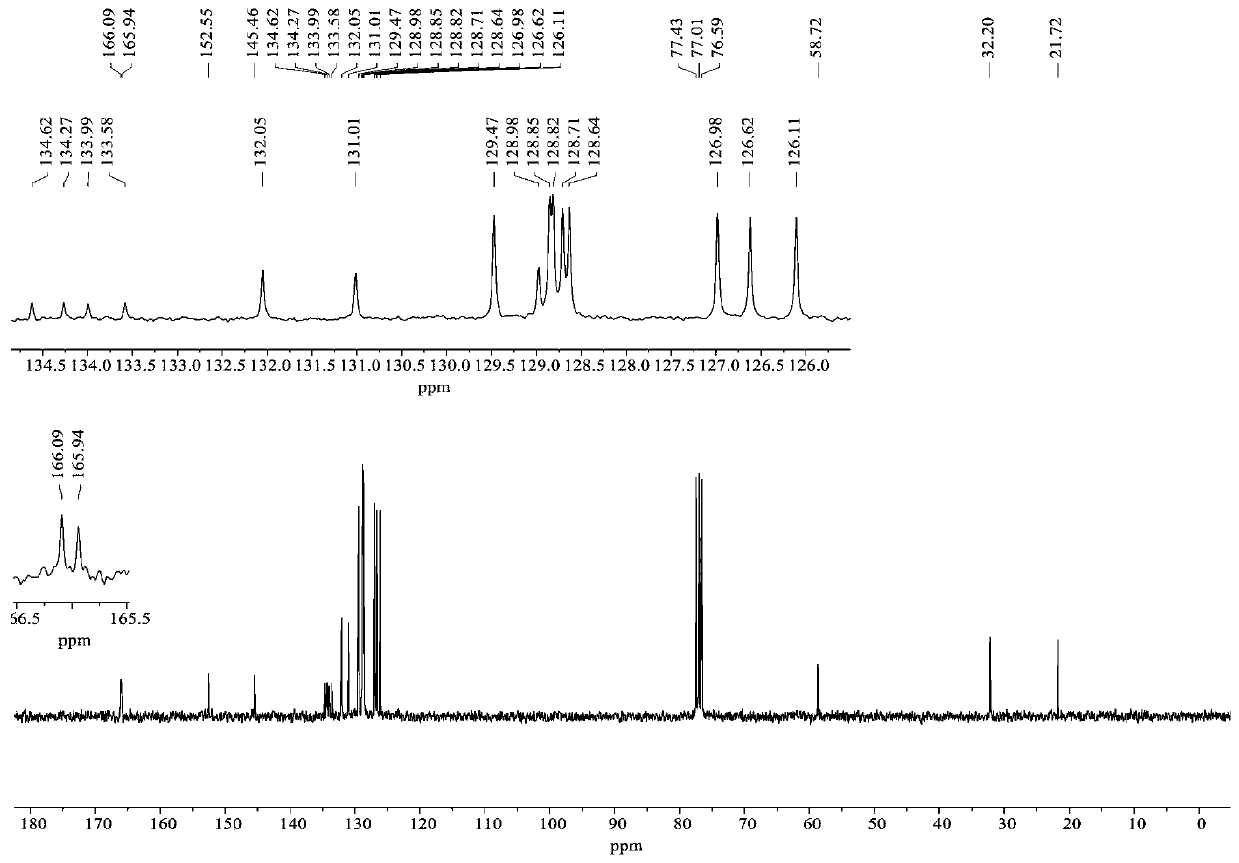
[0045]Synthesis of compound 4b: Take a dry hard reaction tube, add α-bromohydrazone 1b (35.2 mg, 0.1 mmol) into the reaction tube, add azlactone 2a (35.6 mg, 0.15 mmol), and then add anhydrous carbonic acid Potassium (13.8mg, 0.1mmol), finally added anhydrous chloroform (2mL), replaced nitrogen, reacted at room temperature for 24h under the protection of nitrogen. After the reaction was completed, the solvent was concentrated under reduced pressure, separated and purified by column chromatography (petroleum ether / ethyl acetate=10 / 1-3 / 1) to obtain compound 4b. White solid, yield 81%; m.p.:221.8-222.0℃. 1 H NMR (300MHz, CDCl 3 )δ7.95(d, J=7.9Hz, 2H), 7.88(d, J=6.5Hz, 2H), 7.75-7.71(m, 3H), 7.64(t, J=7.9Hz, 1H), 7.53- 7.38(m, 8H), 7.22-7.18(m, 1H), 7.11-7.09(m, 4H), 4.94(d, J=17.5Hz, 1H), 3.47(d, J=17.4Hz, 1H). 13 C NMR (75MHz, CDCl 3 )δ166.1, 166.0, 152.8, 136.9, 134.5, 134.2, 133.5, 132.1, 131.1, 129.1, 128.90, 128.87, 128.65, 128.64, 127.0, 126.6, 126.0, 58.8, ...
Embodiment 3
[0047]
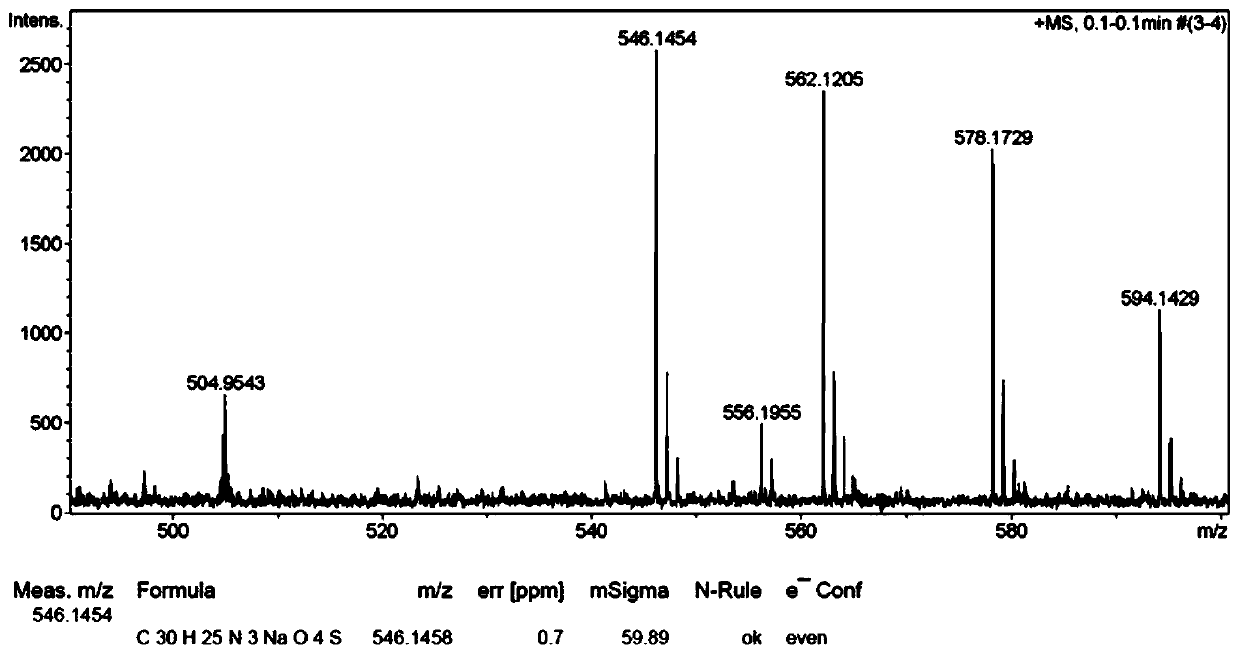
[0048] Synthesis of compound 4c: Take a dry hard reaction tube, add α-chlorohydrazone 1c (35.2 mg, 0.1 mmol) into the reaction tube, add azlactone 2a (35.6 mg, 0.15 mmol), and then add anhydrous carbonic acid Potassium hydrogen (10.0 mg, 0.1 mmol), and finally anhydrous toluene (2 mL) was added to replace the nitrogen, and the reaction was carried out at room temperature under the protection of nitrogen for 24 h. After the reaction was completed, the solvent was concentrated under reduced pressure, separated and purified by column chromatography (petroleum ether / ethyl acetate=10 / 1-3 / 1) to obtain compound 4c. White solid, yield 84%. m.p.:189.0-191.5℃. 1 H NMR (300MHz, CDCl 3 )δ7.88-7.85 (m, 2H), 7.80-7.77 (m, 2H), 7.56-7.42 (m, 9H), 7.34-7.32 (m, 3H), 4.70 (d, J=17.4Hz, 1H) , 3.79(d, J=17.5Hz, 1H), 3.39(s, 3H). 13 C NMR (75MHz, CDCl 3 )δ166.6, 166.5, 153.1, 134.9, 134.2, 133.5, 132.2, 131.1, 129.6, 129.4, 128.9, 128.7, 127.0, 126.5, 126.2, 59.2, 41.3, 32.7. HRM...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com