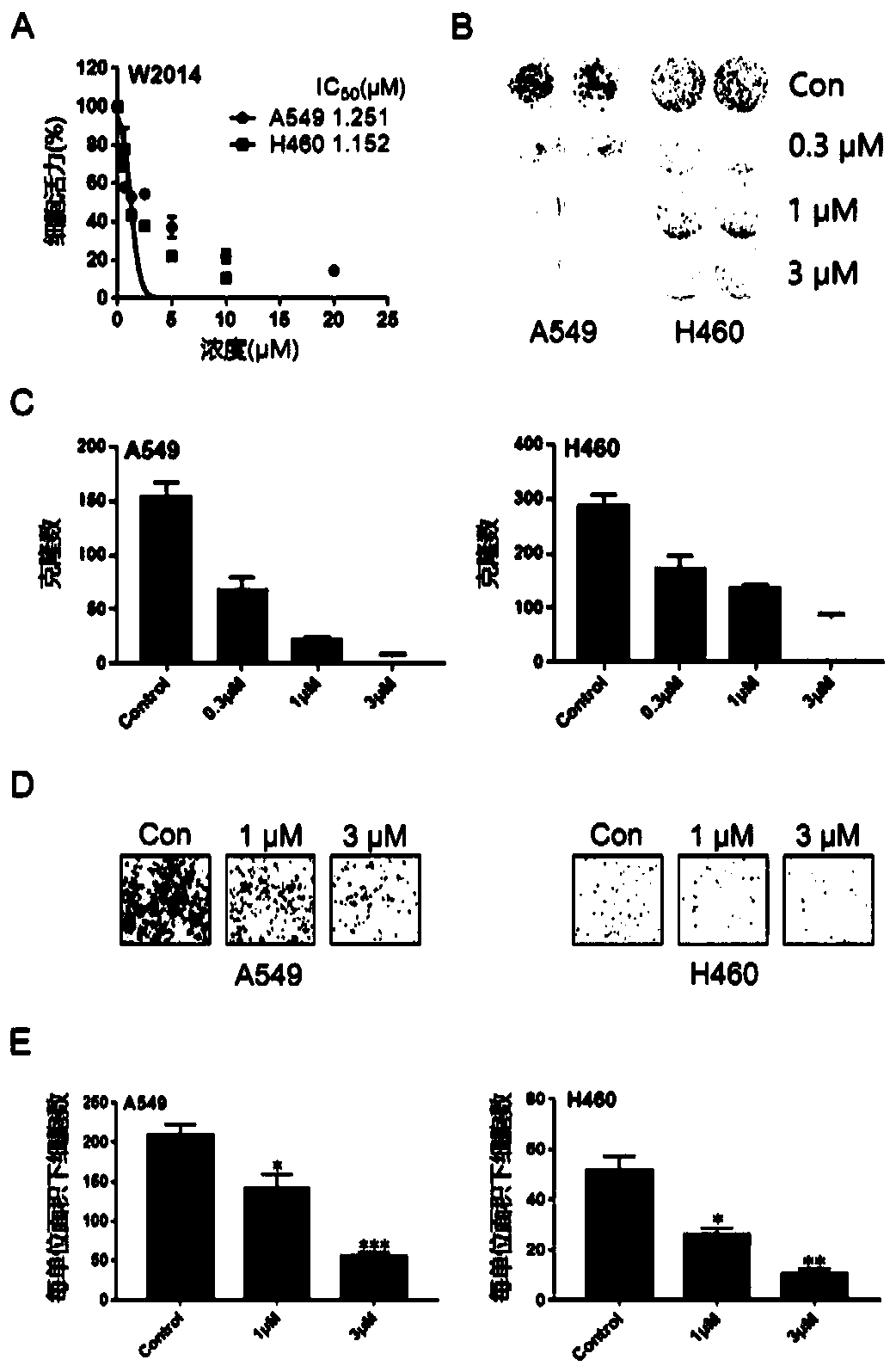
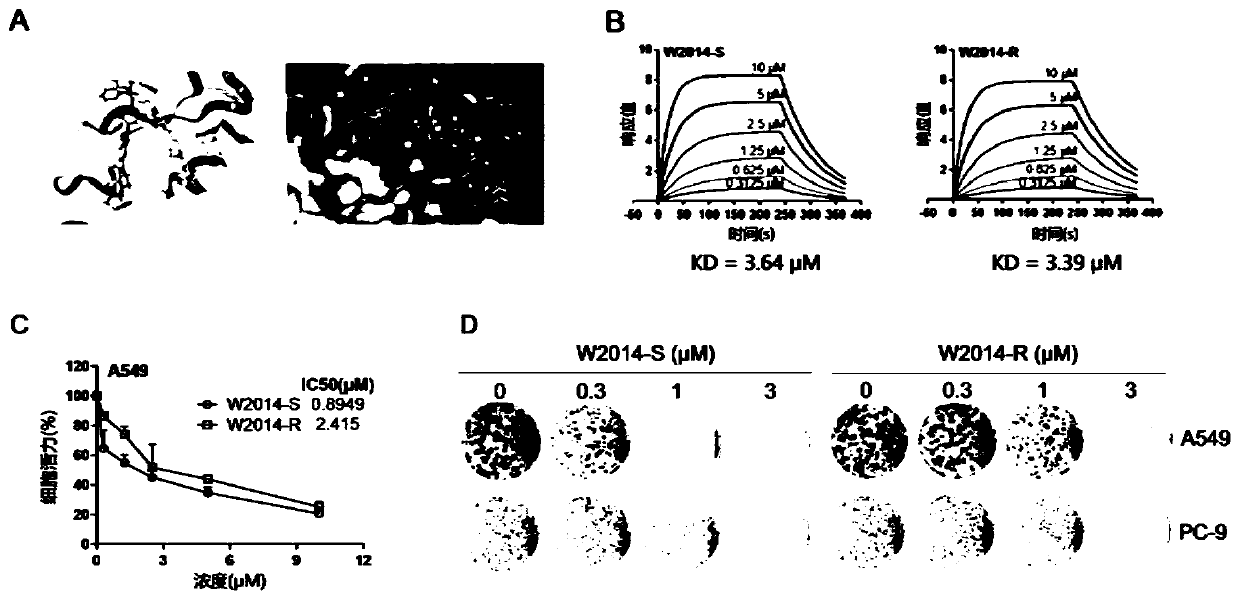
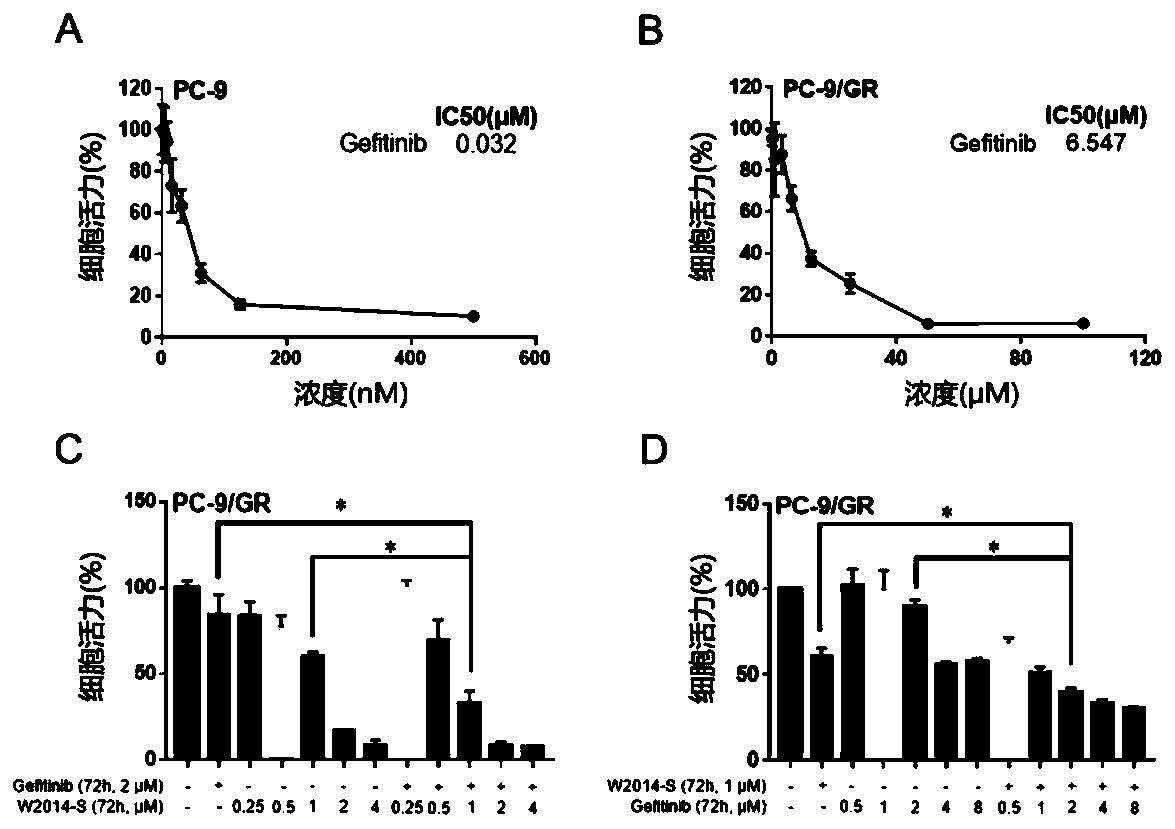
Application of pyrimidazole-type STAT3 inhibitor in preparation of medicine capable of delaying or reversing acquired resistance to TKIs
A pyridoimidazole and inhibitor technology, applied in the field of medicine, can solve the problems of high phosphorylation expression, short survival period, poor prognosis, etc., and achieve the effects of enhancing sensitivity, improving curative effect, and overcoming easy drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Preparation of W2014
[0045]
[0046] Weigh compound 1, iron powder (3 equivalents) and ammonium chloride (4 equivalents) and dissolve them in 20ml of ethanol, heat at 80°C, cool to room temperature after the reaction is complete, filter the reaction solution through diatomaceous earth and rinse the silicon with an appropriate amount of ethyl acetate. alginate, the organic phase was dried with anhydrous sodium sulfate, and spin-dried to obtain a purple solid compound 2 with a yield of 78%. 1 H NMR (500MHz, CDCl 3 )δ6.85(d, J=7.8Hz, 1H), 6.60(d, J=7.8Hz, 1H), 4.45(s, 2H), 3.28(s, 2H).ESI-MS: 145.0[M+H ]+;
[0047] Weigh compound 3, p-hydroxybenzaldehyde (1.1 equivalents), and potassium carbonate (2 equivalents) and dissolve them in 40ml of acetonitrile, stir at room temperature overnight for reaction, filter after the reaction is complete, spin the organic phase to dry column chromatography and purify to obtain compound 4, the yield 90%, 1 H NMR (400MHz, CDCl 3 ...
Embodiment 2
[0050] Embodiment 2: Preparation of W2014R
[0051]
[0052] Weighed triphenylphosphine (1.8 equivalents) and dissolved it in 80 ml of tetrahydrofuran, under nitrogen protection, slowly added DIAD (1.8 equivalents) to the reaction under ice bath conditions, and after 20 minutes, kept ice bath conditions, successively added compound 6 (1 equivalent) and p-Hydroxybenzaldehyde (1 equivalent) were dissolved in 20 ml of tetrahydrofuran and added to the reaction respectively, stirred at room temperature for the reaction, and the reaction was complete after 10 hours. The organic phase was spin-dried and concentrated, and the residue was purified by column chromatography to obtain compound 7 , with a yield of 44%, 1 H NMR (400MHz, CDCl 3 )δ9.84(s,1H),7.78–7.74(m,2H),7.39–7.36(m,4H),7.32–7.28(m,1H),6.99(d,J=8.7Hz,2H),5.44 (q, J=6.4Hz, 1H), 1.70 (d, J=6.4Hz, 3H).ESI-MS: 227.2[M+H]+;
[0053] Weigh intermediate compound 2 and compound 7 (1.1 equivalent) and dissolve in 10ml methano...
Embodiment 3
[0055] Embodiment 3: Preparation of W2014S
[0056]
[0057]Weighed triphenylphosphine (1.8 equivalents) and dissolved it in 80 ml of tetrahydrofuran, under nitrogen protection, slowly added DIAD (1.8 equivalents) to the reaction under ice bath conditions, and after 20 minutes, maintained ice bath conditions, successively added compound 9 (1 equivalent) and p-Hydroxybenzaldehyde (1 equivalent) were dissolved in 20 milliliters of tetrahydrofuran and added to the reaction respectively, stirred at room temperature, and the reaction was complete after 10 hours. The organic phase was spin-dried and concentrated, and the residue was purified by column chromatography to obtain compound 10 , with a yield of 48%, 1 H NMR (400MHz, CDCl 3 )δ9.84(s,1H),7.79–7.74(m,2H),7.40–7.35(m,4H),7.32–7.28(m,1H),6.99(d,J=8.7Hz,2H),5.44 (q, J=6.4Hz, 1H), 1.70 (d, J=6.4Hz, 3H).ESI-MS: 227.2[M+H]+;
[0058] Weigh intermediate compound 2, compound 10 (1.1 equivalent) and dissolve in 10ml methanol, h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com