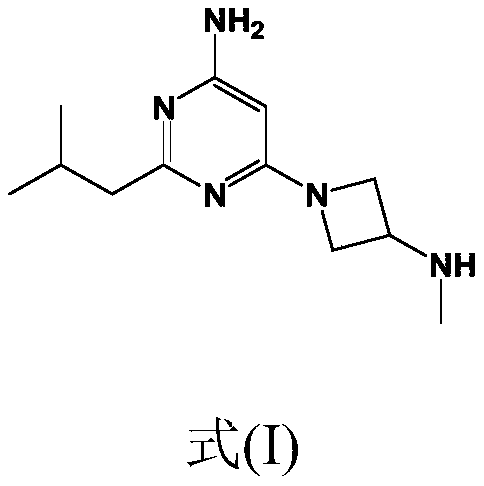
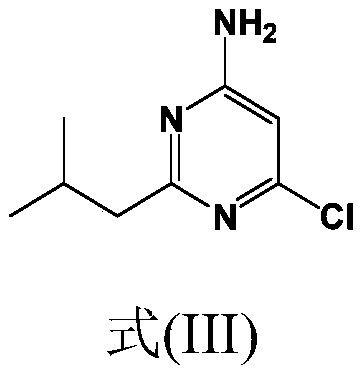
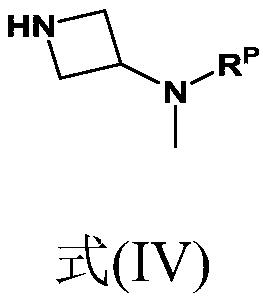
Synthesis of 4-aminopyrimidine compounds
A compound, isobutylpyrimidine technology, applied in the field of organic compound manufacturing, can solve problems such as being unsuitable for modern industrial needs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0219] Example 1: Synthesis of compound (V) [6-amino-2-isobutylpyrimidin-4-ol]
[0220] Reaction: 3-methylbutyramide imide acetate [Compound (VI)] (20.0 g; 125 mmol; 1.0 equivalent) was suspended in EtOH (40 ml; 2 relative volumes). 21% by weight of NaOEt (60.7 g; 187 mmol; 1.5 equivalents) was added and heated to 70°C. A solution of ethyl 2-cyanoacetate [Compound (VII)] (16.2 g; 144 mmol; 1.15 equivalents) (about 4 hours) was slowly added to EtOH (20 ml; 2 volumes). After 40% addition of [Compound (V) 45%] and 100% addition of [Compound (V) 69%], the conversion rate was checked by GC. After reacting at 70°C for 21 hours [85% compound (V)], the conversion rate was checked by GC. The mixture was stirred overnight at 70°C.
[0221] Work-up and separation: The mixture was cooled to 50°C and then quenched with AcOH (0.55 equivalents; 4.13 g). Fill with water (8 volumes). The mixture was concentrated to about 7 volumes at 50°C to 55°C. The suspension was stripped with ACN (3 volum...
Embodiment 2
[0223] Example 2: Synthesis of compound (III) [6-chloro-2-isobutylpyrimidin-4-amine]
[0224] Reaction: The 6-amino-2-isobutylpyrimidin-4-ol [compound (V)] (30.0 g; 179 mmol; 1.0 equivalent) from Example 1 was suspended in ACN (150 ml; 5) at room temperature (RT) Volume). Join POCl 3 (138g; 897mmol; 5.0 equivalents). The suspension was gradually heated to 60°C, 65°C, then 70°C, and stirred at 70°C for 15 minutes. The suspension was further heated to 80°C and stirred overnight. After 19 hours at 80°C, the conversion rate ([Compound (III)] 98.9%) was checked by HPLC.
[0225] Work-up: Concentrate the mixture at 60°C to 70°C to about 3 relative volumes. The mixture was stripped 3 times with toluene (3 volumes) at 60°C to 70°C, diluted with toluene (2 volumes), and then the temperature was adjusted to ±50°C. The mixture was quenched with water (5 volumes). The temperature was adjusted to 60°C, and the mixture was stirred at 55°C to 65°C for at least 30 minutes. Phase separation...
Embodiment 3
[0228] Example 3: Compound (II') [(1-(6-amino-2-isobutylpyrimidin-4-yl)azetidin-3-yl) (formula Base) tert-butyl carbamate] synthesis
[0229] Reaction: The 6-chloro-2-isobutylpyrimidin-4-amine [compound (III)] (25.0 g; 135 mmol; 1.0 equivalent) from Example 2 was combined with K 2 CO 3 (22.3 g; 162 mmol; 1.2 equivalents) was added to the reactor. Add tert-butyl azetidine-3-yl (methyl) carbamate dissolved in dimethyl sulfoxide [Compound (IV')] (26.1 g, 140 mmol, 1.04 equivalent) (m Solution = 148g, 17.6% by weight), the mixture was heated to 110°C. The conversion rate was measured after 24 hours (compound (II') 93%).
[0230] Workup and separation: the mixture was cooled to 50°C to 60°C, then diluted with water (6 volumes) and EtOAc (10 volumes) and stirred for 5 minutes. Phase separation: Separate OL, then wash with water (2 volumes). Concentrate OL to about 6 relative volumes at 65°C to 75°C. The solution was stripped 3 times with iPAc (4 volumes). The solution was slowly c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com