Early diagnosis and treatment biomarker for alveolar echinococcosis and application thereof
A technology of alveolar echinococcosis and targets, applied in the field of biomarkers for early diagnosis and treatment of alveolar echinococcosis, can solve problems such as difficult to effectively diagnose early alveolar echinococcosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1, proteomics analysis
[0050] The samples used in this example were isolated liver and plasma samples from five participants clinically diagnosed with alveolar echinococcosis (AE) and five normal participants (without alveolar echinococcosis) of plasma samples. This protocol has been approved by the Research Ethics Committee of the Affiliated Hospital of Qinghai University, and informed consent has been obtained from each subject.
[0051] 1. Pathological evaluation
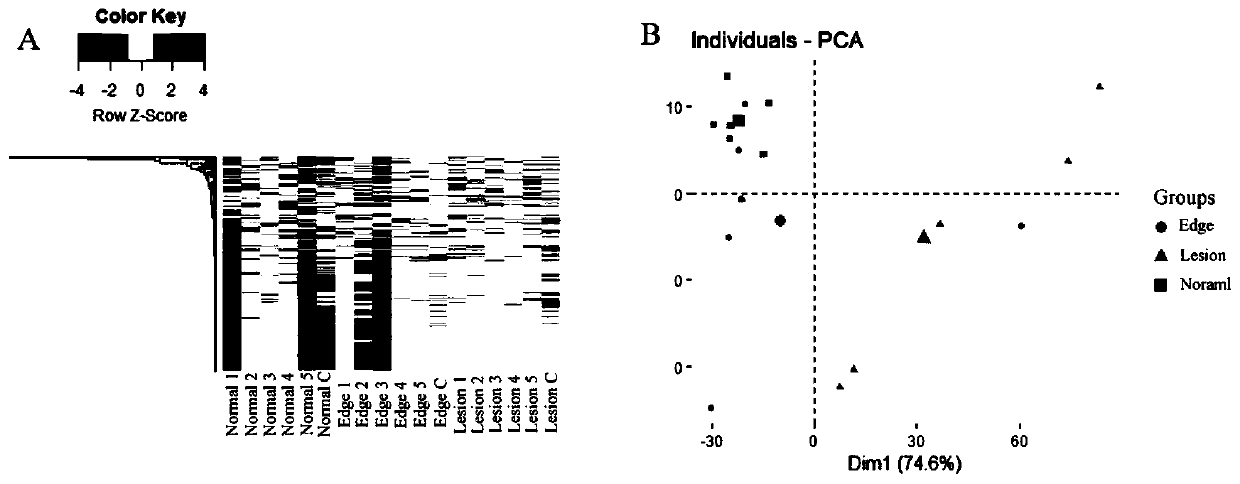
[0052] Isolated livers from participants with alveolar echinococcosis (AE) were divided into three groups: (1) normal tissue of the liver (Normal); (2) liver lesion tissue (Lesion); (3) lesion tissue The edge part (Edge), located between normal and diseased. Histological examination showed no alveolar Echinococcus invasion in the normal part ( figure 1 A, D), but tissue lesions were found in liver fibrous tissue ( figure 1 B, C, D).
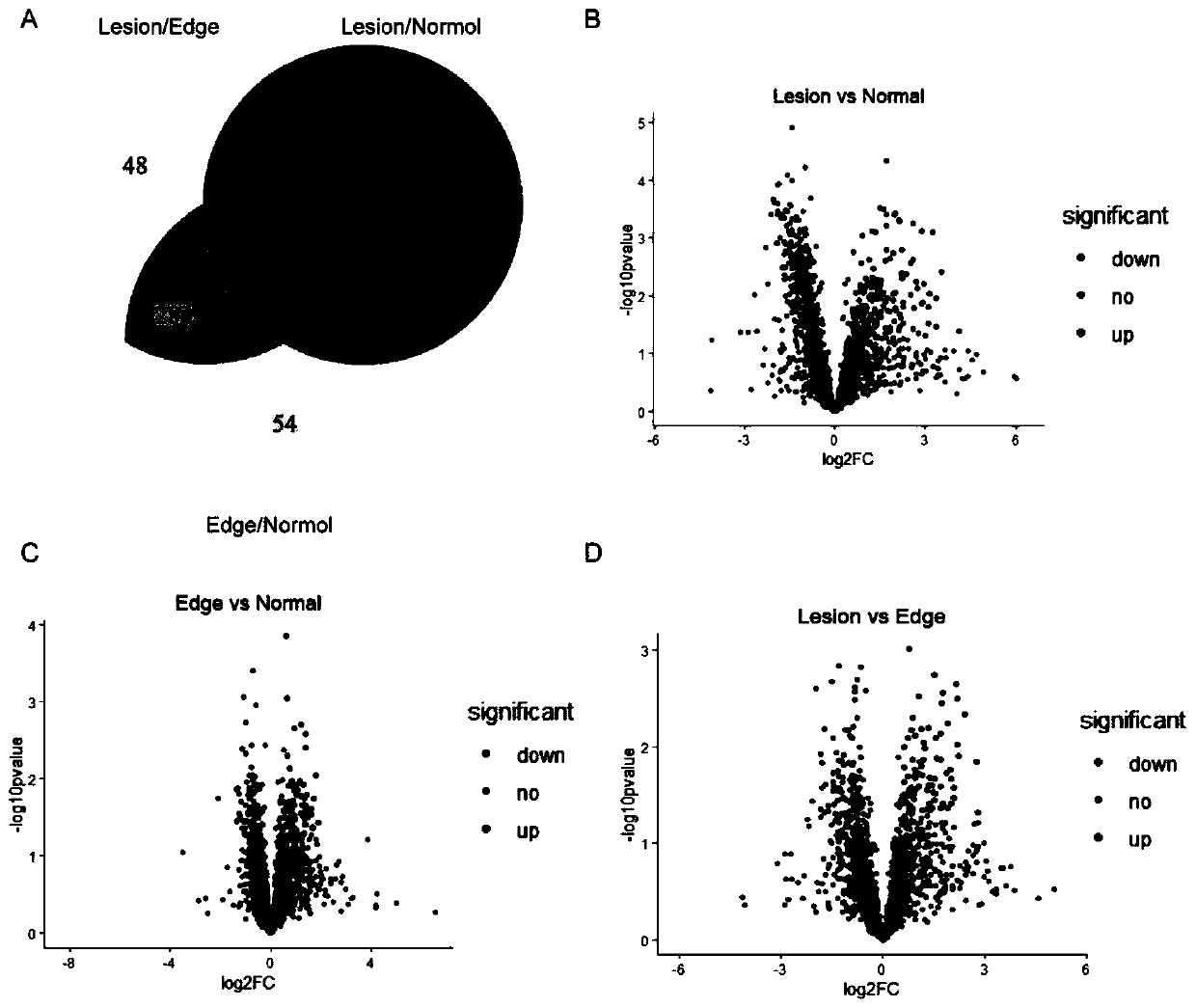
[0053] 2. Protein expression profile in liver tissue ...
Embodiment 2
[0083] Example 2, verification of markers
[0084] The samples in this example are plasma samples from fifty-six clinically confirmed AE patients and fifty-five healthy controls (without alveolar echinococcosis). None of the subjects had a history of respiratory or cardiovascular disease, such as chronic obstructive pulmonary disease, asthma, infectious disease, congenital heart disease, or hypertensive heart disease. Informed consent has been obtained from each subject.
[0085] Plasma samples were incubated at room temperature for 20 minutes, then centrifuged at 3000 rpm for 20 minutes, the supernatant was transferred to a new tube, and the samples were stored at -80°C for further ELISA analysis.
[0086] ELISA analysis was performed using commercial kits according to the instructions.
[0087] ALDH1A1 detection kit was purchased from Shanghai Jianglai Biotechnology Co., Ltd., item number: JL19746.
[0088] TAGLN2 detection kit was purchased from Shanghai Jianglai Biotech...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com