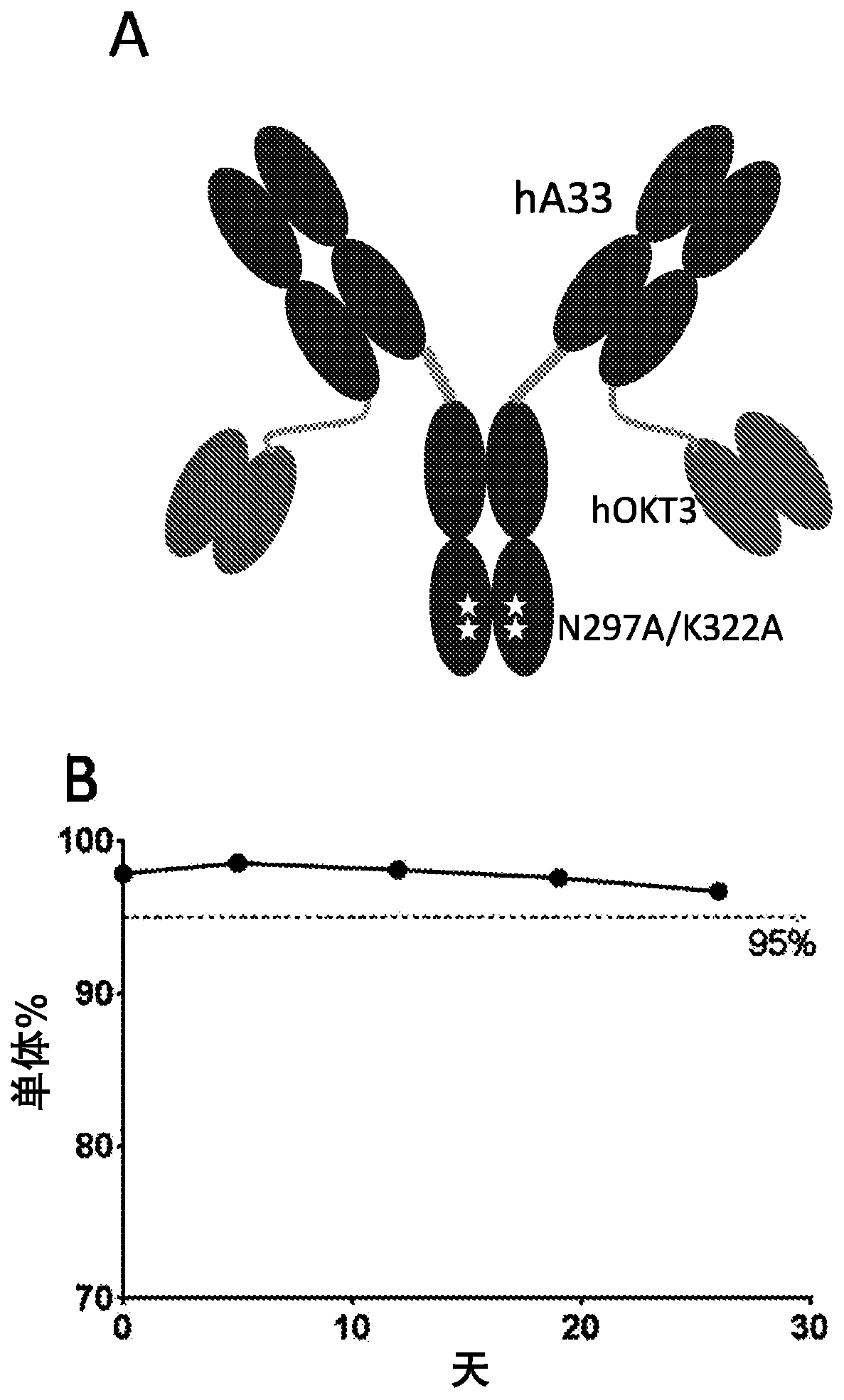
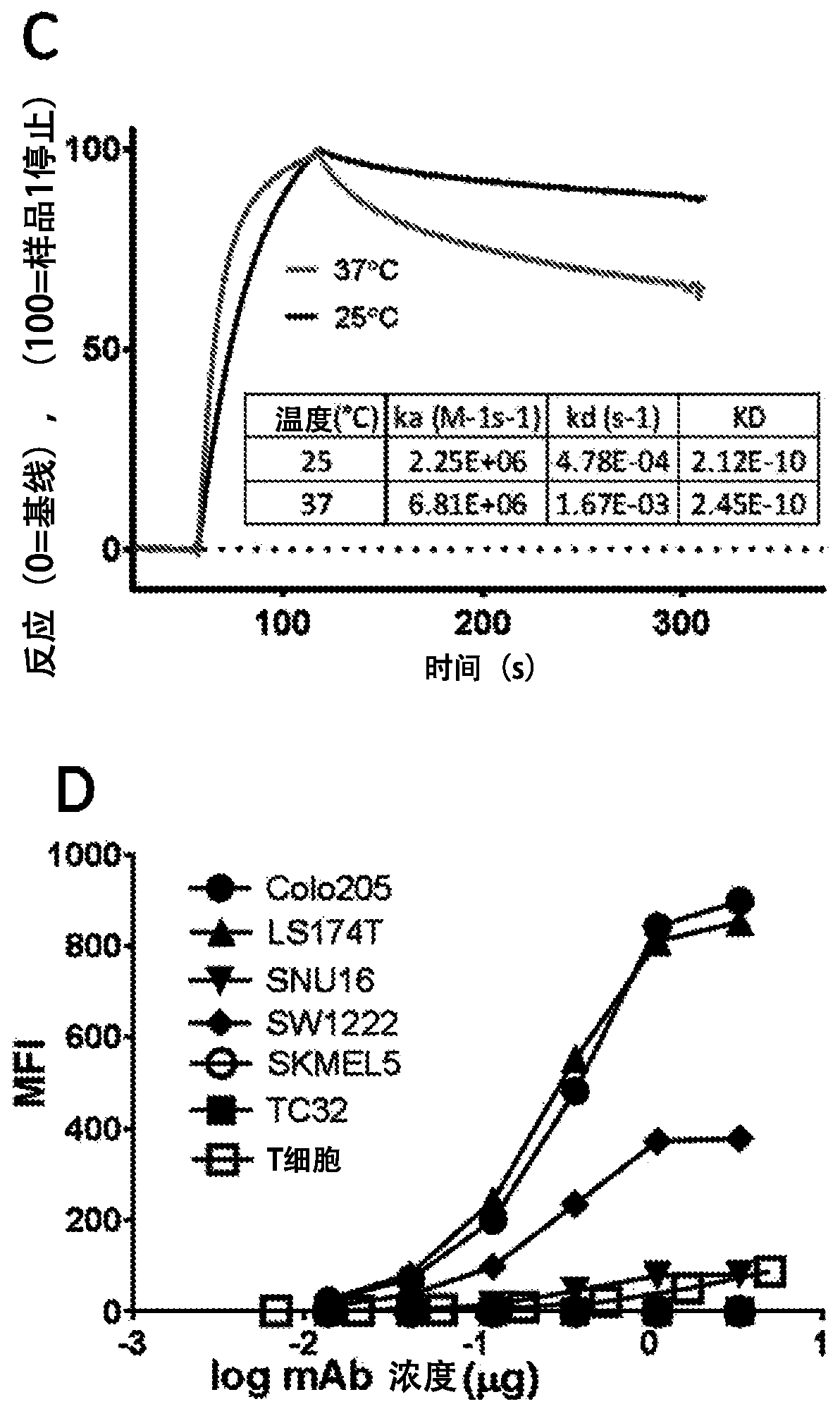
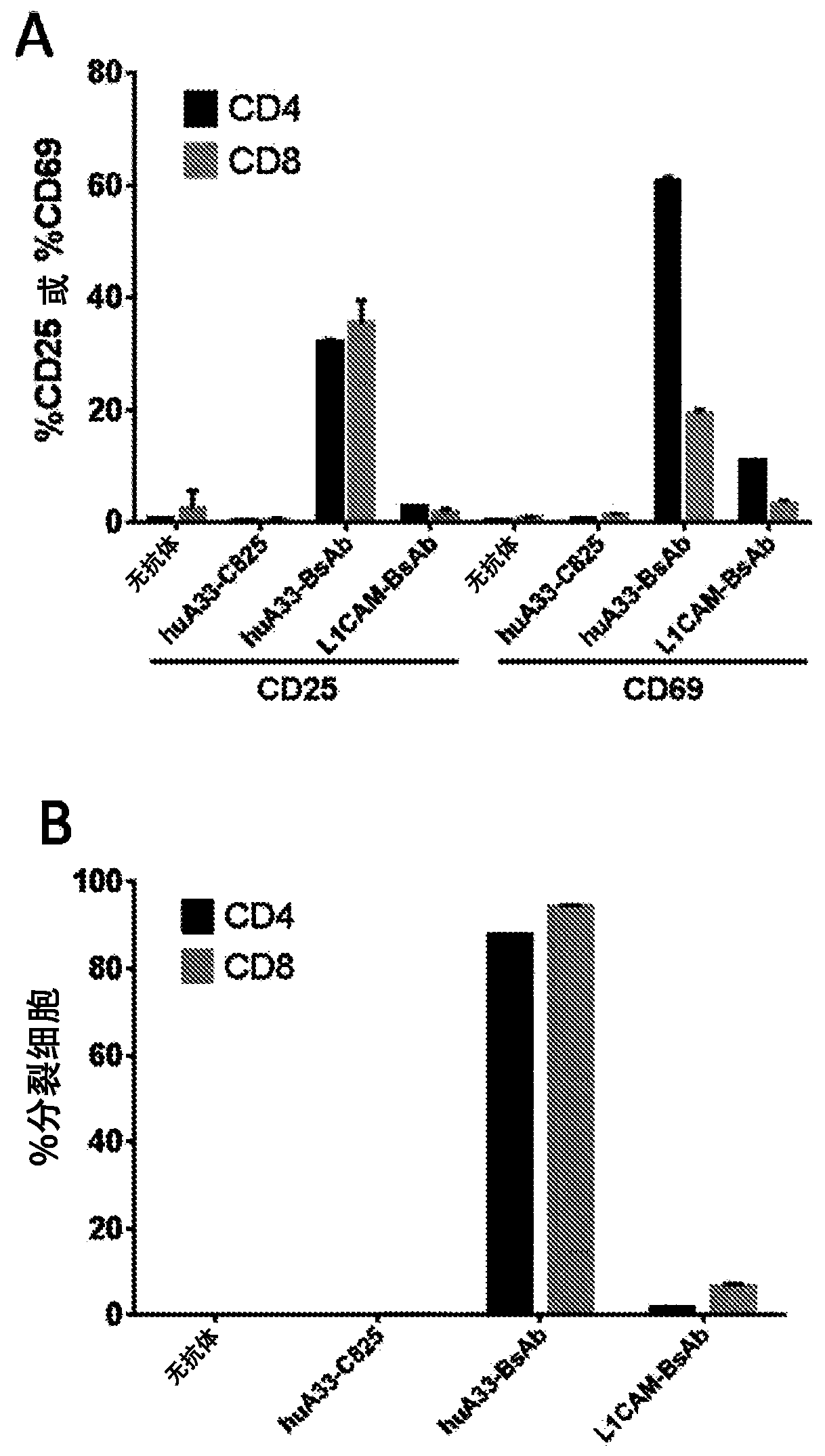
A33 antibody compositions and methods of using the same in radioimmunotherapy
An antibody and immunoglobulin technology, which can be used in in vivo radioactive preparations, antibody mimics/scaffolds, preparations for in vivo experiments, etc., and can solve the problems of limited anti-tumor effect and dose escalation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0180] Preparation of polyclonal antisera and immunogens. Methods of producing antibodies or antibody fragments of the present technology generally comprise immunizing a subject (usually a non-human subject such as a mouse or rabbit) with purified A33 protein or fragment thereof or with cells expressing the A33 protein or fragment thereof . Suitable immunogenic preparations may contain, for example, recombinantly expressed A33 protein or chemically synthesized A33 peptide. Using standard techniques for polyclonal and monoclonal antibody preparation, the ECM of the A33 protein, or a portion or fragment thereof, can be used as an immunogen to generate anti-A33 antibodies that bind to the A33 protein, or a portion or fragment thereof.
[0181] The full length A33 protein or a fragment thereof can be used as an immunogen as a fragment. In some embodiments, the A33 fragment comprises at least five to eight consecutive amino acid residues of the amino acid sequence of SEQ ID NO:57, ...
Embodiment 1
[0330] Example 1: Materials and Methods for Producing and Characterizing Anti-A33 Antibodies of the Present Technology
[0331] Cell lines and human leukocytes. Cell lines LS174T, Colo205, SNU-16 and 293T cells were purchased from ATCC (Manassas, VA); SW1222 was from ECACC (Salisbury, UK). All cells were verified by STR typing. Cells were maintained in 10% FBS (Sigma, St. Louis, MO), 0.03% L-glutamine (GibcoLaboratories, Gaithersburg, MD) and Pen / Strep (Gibco Laboratories, Gaithersburg, MD) supplemented with 10% FBS (Sigma, St. Louis, MO). ) in RPMI medium. Buffy coats from healthy donors were purchased from New York Blood Center (New York City, NY) and human PBMCs were isolated by Ficoll gradients of buffy coats.
[0332] Establishment of cell lines expressing luciferase. 293T cells were first transfected with a retroviral construct containing the luciferase and GFP genes using PolyJet transfection reagent (SignaGen, Rockville, MD) according to the manufacturer's instru...
Embodiment 2
[0348] Example 2: Structure and Binding Affinity of Humanized Anti-A33 Antibodies of the Present Technology
[0349] Figure 14 The V of the murine A33 antibody is shown H and V L Amino acid sequences of domains and their corresponding homologous human sequences (SEQ ID NO: 1-4). The V of the 3A3-H1 / L1, 3A3-H1 / L2, 3A3-H2 / L1 and 3A3-H2 / L2 humanized A33 antibodies of the present technology H and V L The amino acid sequence of the domain is in Figure 15 and Figure 17 shown in . V of the 3A3-H1 / L1, 3A3-H1 / L2, 3A3-H2 / L1 and 3A3-H2 / L2 humanized A33 antibodies H and V L The cDNA sequence of the domain is in Figure 16 and Figure 18 shown in . Figure 19 An alignment of the original humanized amino acid sequence hA33 from King et al. (1995) supra with the newly rehumanized huA33 (3A3) amino acid sequence is shown.
[0350] Figure 24 The amino acid sequences of the light and heavy chains of chimeric chA33-IgG1 are shown, corresponding to SEQ ID NO: 13 and SEQ ID NO: ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com