A kind of high-brightness, high-stability fluorescent dye excited by 488nm and its synthesis method
A fluorescent dye and high-stability technology, applied in the field of fluorescent dyes, can solve the problems that fluorescent dyes cannot meet the needs of fluorescence imaging and detection, fluorescence signal reading errors, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Synthesis of N-butyl-4,5-diazetidinyl-1,8-naphthalimide (BuAN-DAze):
[0045] Synthesis of intermediate N-butyl-4-bromo-5-nitro-1,8-naphthalimide (BuAN-NBr):
[0046]
[0047]4-Bromo-5-nitro-1,8-naphthalimide (1.0 g, 3.11 mmol) was dissolved in 20 mL of ethanol, and n-butylamine (250 mg, 3.43 mmol) was added dropwise thereto. After 1 h at 70°C, the solvent was distilled off under reduced pressure, and the residue was separated through a silica gel column (petroleum ether: dichloromethane = 2:1, V / V) to obtain 620 mg of off-white solid with a yield of 53%.
[0048] 1 H NMR (400MHz, CDCl 3 )δ8.72(d, J=7.8Hz, 1H), 8.52(d, J=7.9Hz, 1H), 8.21(d, J=7.9Hz, 1H), 7.95(d, J=7.8Hz, 1H) ,3.66(t,J=6.5Hz,2H),1.68(m,2H),1.40(m,J=7.8Hz,2H),0.94(t,J=7.9Hz,3H).
[0049] Synthesis of N-butyl-4,5-diazetidinyl-1,8-naphthalimide (BuAN-DAze):
[0050]
[0051] Dissolve N-butyl-4-bromo-5-nitro-1,8-naphthalimide (100mg, 0.26mmol) in 20mL of ethylene glycol methyl ether, and add azeti...
Embodiment 2
[0059] Synthesis of N-butyl-4,5-bis(azacyclopentyl)-1,8-naphthalimide (BuAN-DAzo):
[0060]
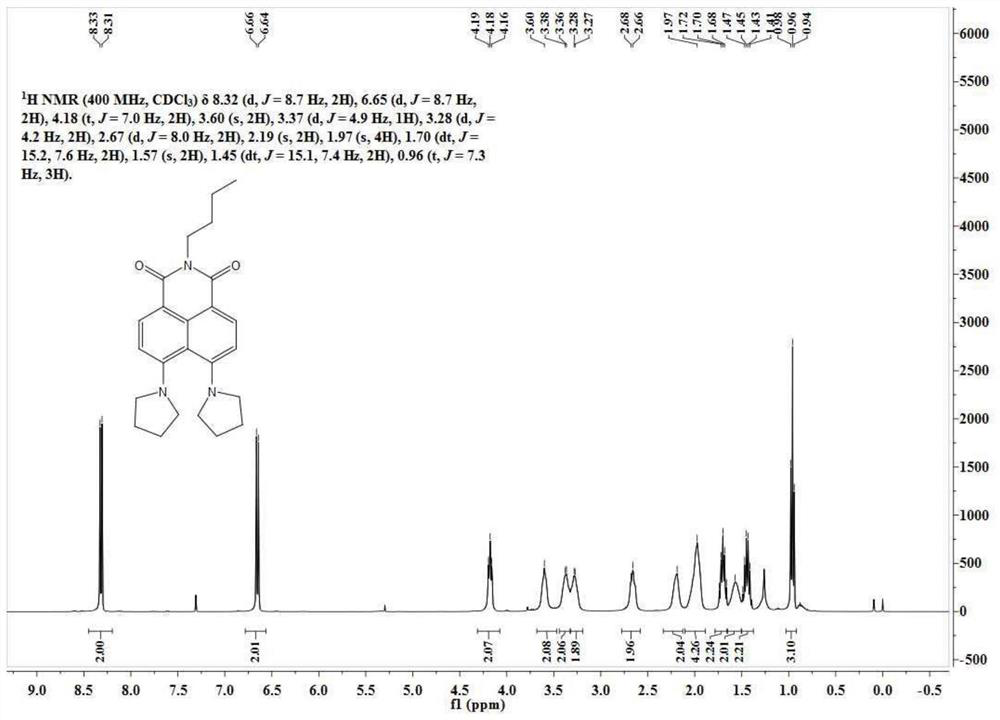
[0061] N-butyl-4-bromo-5-nitro-1,8-naphthalimide (50 mg, 0.13 mmol) was dissolved in 5 mL of ethylene glycol methyl ether, and 200 mg of tetrahydropyrrole was added thereto. The reaction solution was slowly heated to 140°C and reacted for 10h. Ethylene glycol methyl ether was removed under reduced pressure, and the residue was separated through a silica gel column (dichloromethane:methanol=100:1, V / V) to obtain 38 mg of a yellow solid with a yield of 75%. The NMR spectrum and carbon spectrum of the BuAN-DAzo prepared in Example 2 are as follows: image 3 , 4 As shown, the specific data are:
[0062] 1 H NMR (400MHz, CDCl 3 )δ8.32(d, J=8.7Hz, 2H), 6.65(d, J=8.7Hz, 2H), 4.18(t, J=7.0Hz, 2H), 3.60(s, 2H), 3.37(d, J=4.9Hz, 1H), 3.28(d, J=4.2Hz, 2H), 2.67(d, J=8.0Hz, 2H), 2.19(s, 2H), 1.97(s, 4H), 1.70(dt, J=15.2,7.6Hz,2H), 1.57(s,2H),1.45(dt,J=15.1,7.4Hz,2H),0.96(t,J=7.3Hz,3H). ...
Embodiment 3
[0070] Synthesis of N-butyl-4,5-ethylenediamino-1,8 naphthalimide (BuAN-EDA):
[0071]
[0072] N-butyl-4-bromo-5-nitro-1,8-naphthalimide (100 mg, 0.27 mmol) was dissolved in 25 mL of ethylene glycol methyl ether, and 100 mg of ethylenediamine was added thereto. The reaction solution was slowly heated to 50°C and reacted for 24h. Ethylene glycol methyl ether was removed under reduced pressure, and the residue was separated through a silica gel column (dichloromethane:methanol=70:1, V / V) to obtain 71 mg of a yellow solid with a yield of 87%. The NMR spectrum hydrogen spectrum of the BuAN-EDA prepared by embodiment 3 is as Figure 5 As shown, the specific data are:
[0073] 1 H NMR (400MHz, DMSO-d 6 )δ8.29(s,2H),8.03(d,J=8.6Hz,2H),6.67(d,J=8.7Hz,2H),4.01–3.92(m,2H),3.51(s,4H), 1.54(dt,J=14.9,7.5Hz,2H),1.31(dt,J=14.8,7.4Hz,2H),0.90(t,J=7.3Hz,3H). 13 C NMR (101MHz, DMSO-d 6 )δ163.36, 155.59, 135.28, 133.35, 110.27, 107.36, 105.79, 46.73, 38.97, 30.40, 20.35.
[0074] Af...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorption wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com