Dihydrofuran carboxamides
A kind of technology of dihydrofuran and carboxamide, applied in the field of novel dihydrofuran-carboxamide
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
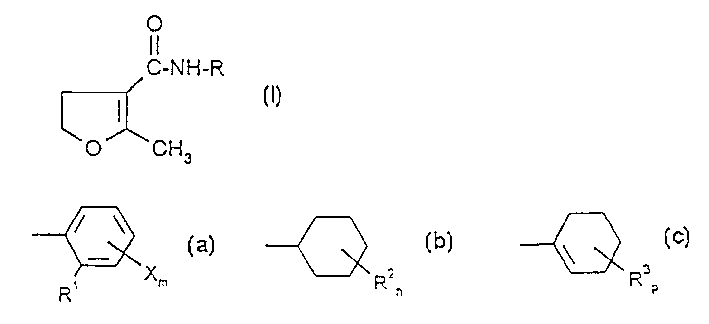
[0121] At room temperature, a solution of 1.5 g (0.01 mol) of 2-methyl-4,5-dihydrofuran-3-carbonyl chloride in 10 ml of toluene was added dropwise while stirring to 2.2 g (0.01 mol) of 4-fluoro- In a mixture of 2-cyclooctylaniline, 1.0 g of triethylamine and 40 ml of toluene. After the addition was complete, the reaction mixture was stirred at room temperature for an additional 2 hours. The reaction mixture was then mixed with water. The organic phase was separated, dried over sodium sulfate and concentrated under reduced pressure. The remaining residue was stirred with hexanes. The obtained crystalline product was adsorptively filtered and dried under reduced pressure at 59°C. In this way 2.2 g (66.7% of theory) of N-(4-fluoro-2-cyclooctyl)-2-methyl-4,5-dihydrofuran-3-carboxamide were obtained in the form Solid substance with a melting point of 91°C.
[0122] The dihydrofurancarboxamides of general formula (I) listed in Table 1 below were prepared in a similar manner. T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com