A functional recyclable polymer homopolymer and its preparation method and application
A homopolymer and polymer technology, applied in the field of functional recyclable polymer homopolymer and its preparation, can solve the problems of increased production cost, complex process, complex composition, etc., and achieve low cost, wide source and simple operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
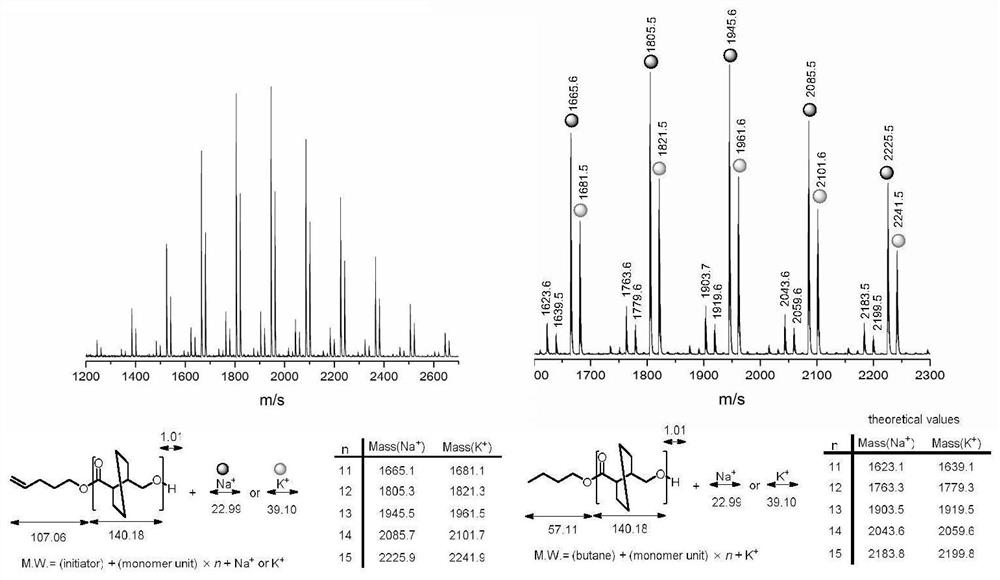
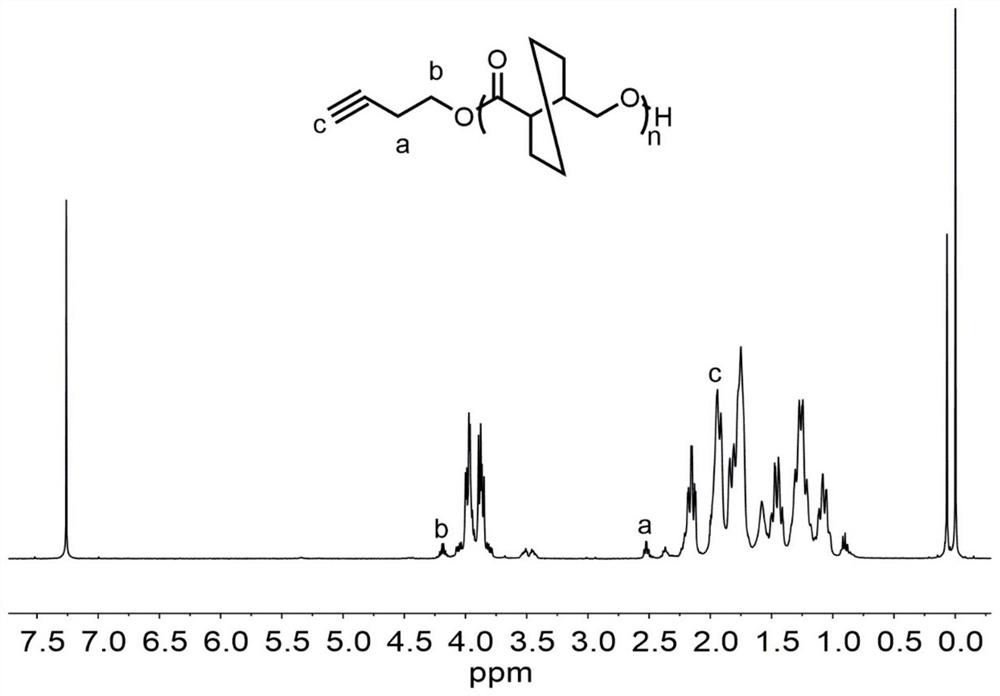
[0066] Diphenylmethanol (0.0111 g, 0.06 mmol) and (R)-hexahydro-(3,4)-trans-benzofuranone (0.4205 g, 3 mmol) were added to anhydrous and oxygen-treated In the ampoule, after stirring at 200 rpm for 10 minutes, n-butylethylmagnesium (0.06 ml, 0.06 mmol) was added, and left to react at room temperature for 24 hours. After the reaction is over, add benzoic acid / dichloromethane solution to dissolve the mixture, take it out and add it to a cold methanol solution, and a polymer is precipitated. The white solid was separated by filtration, and transferred to a vacuum oven to dry to obtain a polymer, whose spectrum is shown in Figure 8 . The conversion rate through the reaction solution also 1 Calculated by H NMR, the polymer structure is obtained by 1 H NMR identification, molecular weight and dispersion of the polymer were determined by GPC. The conversion rate was 89%, the number average molecular weight of the polymer was 6550 g / mol, and the dispersion coefficient was 1.02. ...
Embodiment 2
[0068] Add benzphenylmethanol (0.0111g, 0.06 mmol) and γ-butyrolactone (0.5165g, 6 mmol) to an anhydrous anaerobic treated ampoule, add 0.25 ml of toluene, and stir at 200rpm for 10 minutes , added di-n-butylmagnesium (0.12 ml, 0.12 mmol), and reacted at -50°C for 24 hours. After the reaction is over, add benzoic acid / dichloromethane solution to dissolve the mixture, take it out and add it to a cold methanol solution, and a polymer is precipitated. The white solid was separated by filtration, and transferred to a vacuum oven to dry to obtain a polymer, whose spectrum is shown in Figure 9 . The conversion rate through the reaction solution also 1 Calculated by H NMR, the polymer structure is obtained by 1 H NMR identification, molecular weight and dispersion of the polymer were determined by GPC. The conversion rate was 82%, the number average molecular weight of the polymer was 7440 g / mol, and the dispersion coefficient was 1.05.
Embodiment 3
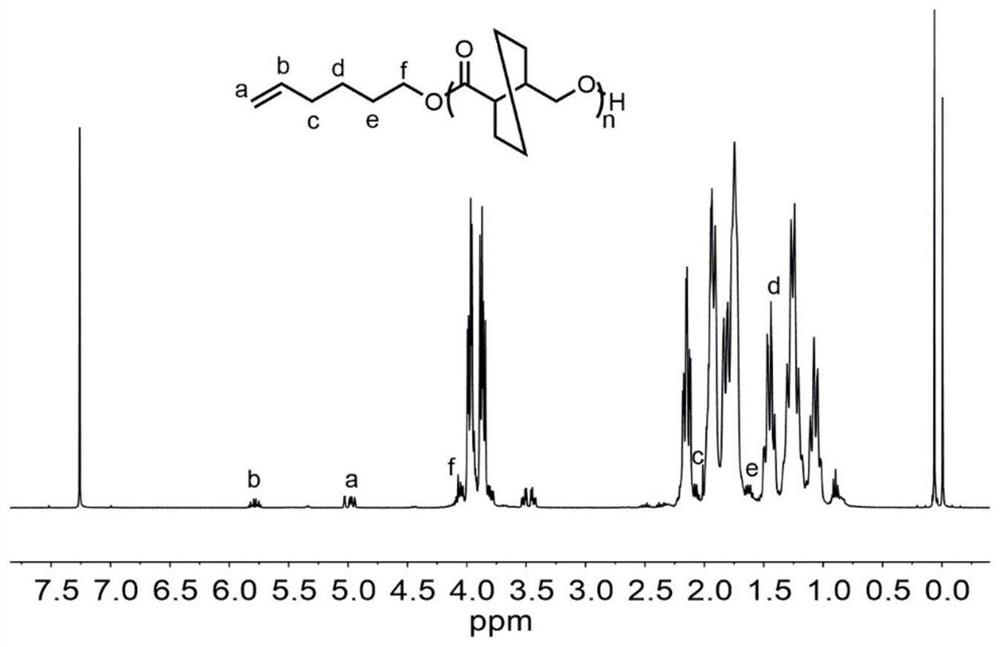
[0070] 5-Hexen-1-ol (0.0060 g, 0.06 mmol) and (R)-hexahydro-(3,4)-trans-benzofuranone (0.2523 g, 1.8 mmol) were added to anhydrous In the oxygen-treated ampoule, after stirring at 200 rpm for 10 minutes, di-n-butylmagnesium (0.03 ml, 0.03 mmol) was added, and the mixture was reacted at room temperature for 12 hours. After the reaction is over, add benzoic acid / dichloromethane solution to dissolve the mixture, take it out and add it to a cold methanol solution, and a polymer is precipitated, which is 5-hexen-1-ol functionalized poly((R)-hexahydro- (3,4)-trans-benzofuranone), whose spectrum is shown in figure 1 and figure 2 . The white solid was separated by filtration and transferred to a vacuum oven for drying to obtain a polymer. The conversion rate through the reaction solution also 1 Calculated by H NMR, the polymer structure is obtained by 1 H NMR identification, molecular weight and dispersion of the polymer were determined by GPC. The conversion rate was 87%, the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com