Method for constructing luciferase high-expressed stable cell line and cell line
A technology of luciferase and construction method, which can be applied in the field of Huh7 cell line, and can solve problems such as difficulties in the treatment of liver cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Example 1 pWPXLd-luciferase virus packaging and infection
[0014] The construction method of the pWPXLd-luciferase plasmid used refers to the master's thesis "Construction of MDA-MB-231 breast cancer cell line stably expressing luciferase and its activity characterization, Zhou Xin, Northeast Normal University".
[0015] 1. Day 1: Place 3x10 6 293T cells were planted in a 10cm dish, cultured in 10ml of DMEM medium supplemented with fetal bovine serum, this medium did not contain antibiotics, placed at 37°C, 5% CO 2 (CO 2 5% air by volume) in an incubator.
[0016] 2. The next day: when the coverage rate of 293T cells reaches 80%, use lipofectamine 2000 for cell transfection.
[0017] The ratio of transfection reagents is as follows:
[0018] Sterile EP tube 1:
[0019]
[0020] Sterile EP tube 2:
[0021]
[0022] After mixing tube 1 and tube 2 evenly, absorb the liquid in tube 2 and put it in tube 1, shake it gently and mix well, then put it in the ultra-c...
Embodiment 2
[0026] The expression situation of luciferase in the monoclonal cell of embodiment 2
[0027] 1. Dissolve D-fluorescein salt (YEASEN 40902ES03) in distilled water and prepare it as a 30mg / ml stock solution (200x).
[0028] 2. Dilute the stock solution 1:200 with the preheated culture medium to prepare the working concentration (150μg / ml).
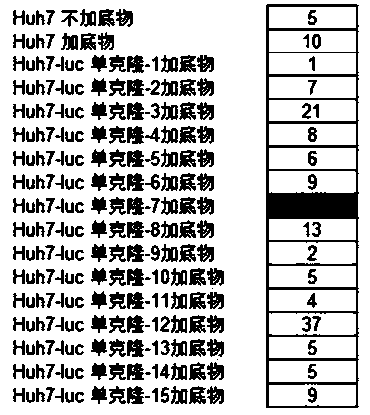
[0029] 3. A total of 17 plates of cells, the original medium in the culture dish was discarded, normal Huh7 cells were treated without fluorescein working solution and normal Huh7 cells were treated with fluorescein working solution as controls, and monoclonal Huh7-luc was picked Cells 1-15 were treated with fluorescein working solution as the experimental group, incubated at 37°C for 10 minutes, and then detected the fluorescence intensity with a BioTek microplate reader. The result is as figure 1 As shown, the selected monoclonal cell 7 is a cell line overexpressing luciferase.
example 3
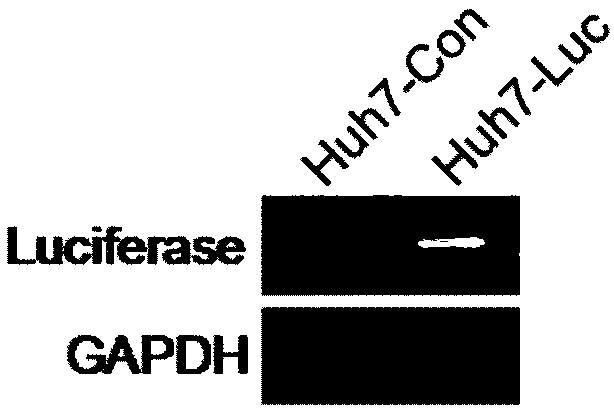
[0030] Example 3 Verification of Huh7-luciferase stable overexpression cell line
[0031] 1. Extract the RNA of Huh7 and Huh7-luciferase cells according to the literature "Targeting KDM1A attenuates Wnt / β-catenin signaling pathway to eliminate sorafenib-resistant stem-like cells in hepatocellular carcinoma, Huang M, Cancer Lett. 2017Jul 10; 398:12-21", and then Reverse transcribed into cDNA and tested by RT-PCR.
[0032] 2. The primer sequences required to detect the Luciferase gene are as follows:
[0033] 5'CTGAACAGCATGGGCATCA 3'(sense)
[0034] 5'AAATGGGAAGTCACGAAGGT 3'(antisense)
[0035] 3. The reaction system is as follows:
[0036] system 25μl DNA template (100ng / μl) 5μl Double primer (2μM) 3μl Es-tag enzyme (Takara RR902Q) 12.5μl wxya 2 o
to 25μl
[0037] 4. Reaction conditions
[0038]
[0039] The result is as figure 2 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com