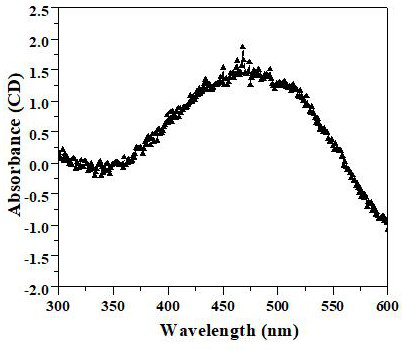
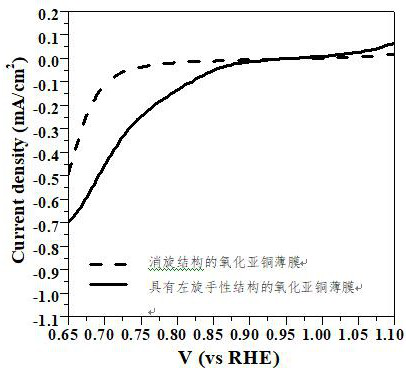
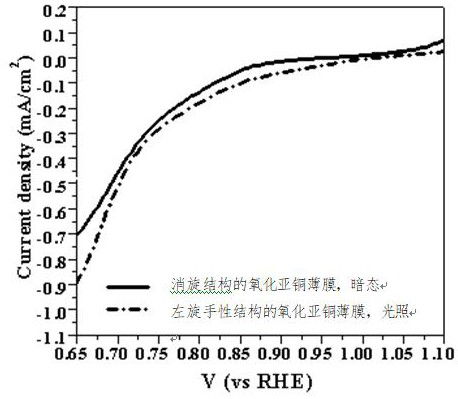
A cuprous oxide film and its application in fuel cells
A cuprous oxide and fuel cell technology, applied in battery electrodes, circuits, electrical components, etc., can solve the problems of high cost of platinum-based catalysts, hindering the commercial development and application of fuel cells, etc., and achieve easy large-scale preparation and scale Production, improved oxygen reduction efficiency, and strong controllability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The preparation of a cuprous oxide thin film has a left-handed chiral structure, and the steps are as follows:
[0036] In the electrolyte, a saturated calomel electrode was used as a reference electrode, a platinum black electrode was used as a counter electrode, and a quartz glass sheet sputtered with a 5nm gold layer on the surface was used as a working electrode, and a constant voltage of -0.5V was applied to the working electrode to deposit Time 5min; During the deposition and growth of the film, the chiral molecules in the electrolyte induce the reactant atoms along the The preferred orientation of the crystal plane grows; after a period of deposition and growth, a cuprous oxide film with a left-handed chiral structure is obtained.
[0037] The electrolyte is L-chiral tartaric acid, CuSO 4 ·5H 2 The mixed solution of O and NaOH, the solvent is deionized water; wherein, the concentration of chiral tartaric acid is 0.2mol / L, CuSO 4 ·5H 2 The concentration of O ...
Embodiment 2
[0042] The preparation of a cuprous oxide thin film has a left-handed chiral structure, and the steps are as follows:
[0043] In electrolyte, with saturated calomel electrode as reference electrode, with platinum black electrode as counter electrode, with gold plate as working electrode, apply-0.6V constant voltage to working electrode, deposition time 20min; During the process, chiral molecules in the electrolyte induce reactant atoms along The preferred orientation of the crystal plane grows; after a period of deposition and growth, a cuprous oxide film with a left-handed chiral structure is obtained.
[0044] The electrolyte is L-chiral tartaric acid, CuSO 4 ·5H 2 The mixed solution of O and NaOH, the solvent is deionized water; wherein, the concentration of chiral tartaric acid is 0.2mol / L, CuSO 4 ·5H 2 The concentration of O is 0.2mol / L, the concentration of NaOH is 3mol / L; 4 ·5H 2 The O solution is mixed evenly so that the Cu 2+ Ions are complexed with chiral ta...
Embodiment 3
[0050] The preparation of a cuprous oxide thin film has a left-handed chiral structure, and the steps are as follows:
[0051] In electrolyte, with saturated calomel electrode as reference electrode, with platinum black electrode as counter electrode, with FTO glass as working electrode, apply-0.7V constant voltage to working electrode, deposition time 30min; During the process, chiral molecules in the electrolyte induce reactant atoms along The preferred orientation of the crystal plane grows; after a period of deposition and growth, a cuprous oxide film with a left-handed chiral structure is obtained.
[0052] The electrolyte is L-chiral tartaric acid, CuSO 4 ·5H 2 The mixed solution of O and NaOH, the solvent is deionized water; wherein, the concentration of chiral tartaric acid is 0.2mol / L, CuSO 4 ·5H 2 The concentration of O is 0.2mol / L, the concentration of NaOH is 3mol / L; 4 ·5H 2 The O solution is mixed evenly so that the Cu 2+ Ions are complexed with chiral tar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com