Fluorescent PCR detection kit for identifying infection and immunization of African swine fever
A technology of African swine fever and African swine fever virus, applied in the field of fluorescent quantitative PCR detection reagents, can solve the problems of ASFV prevention and control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
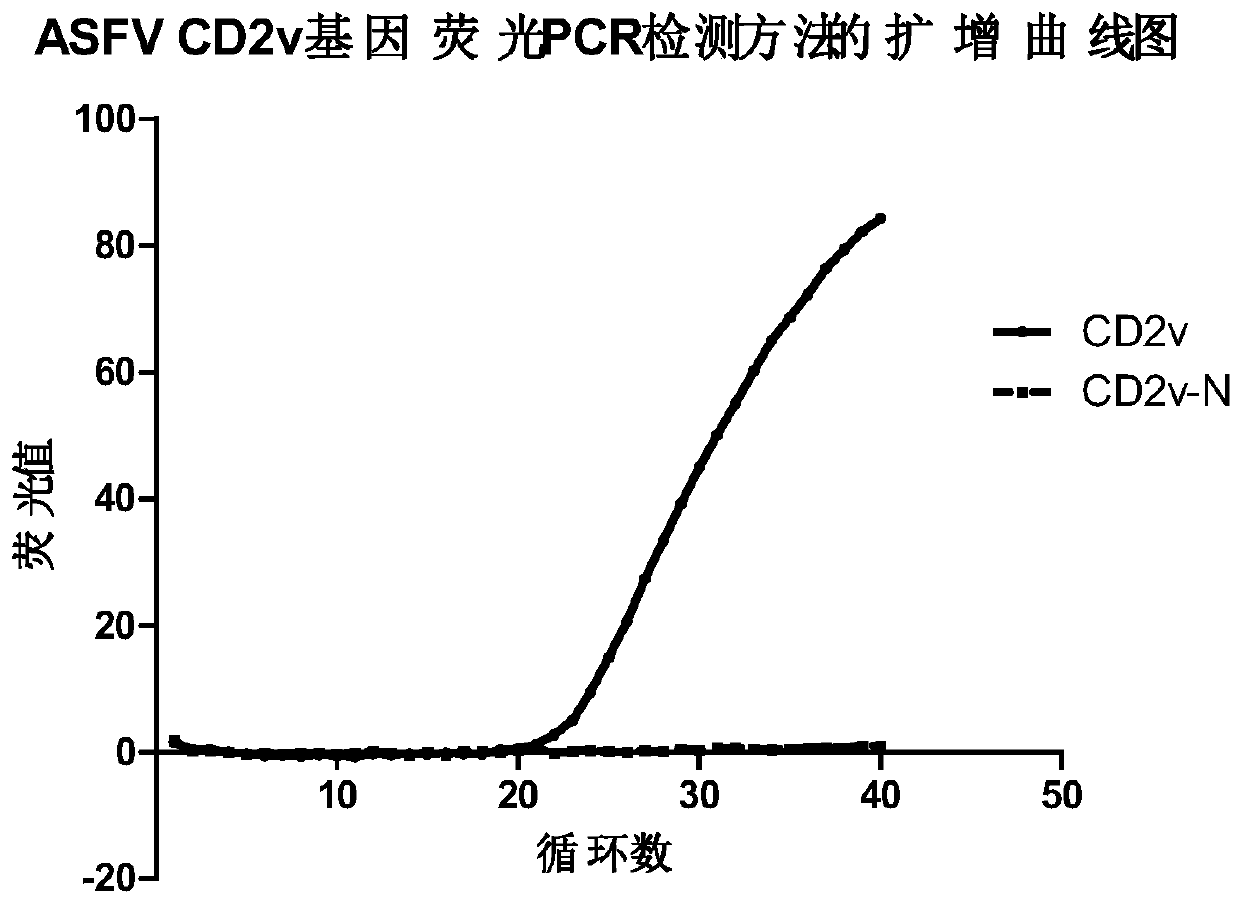
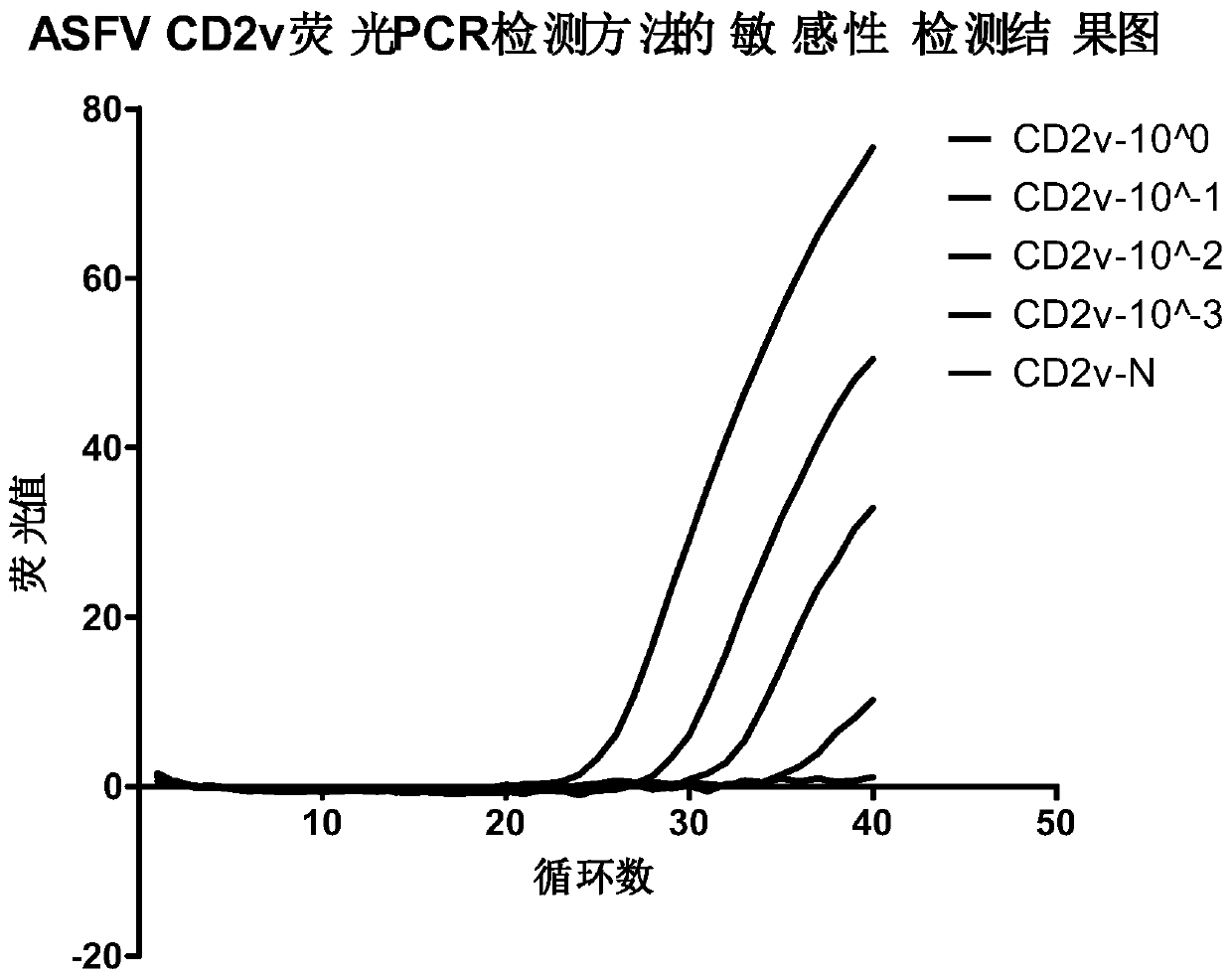
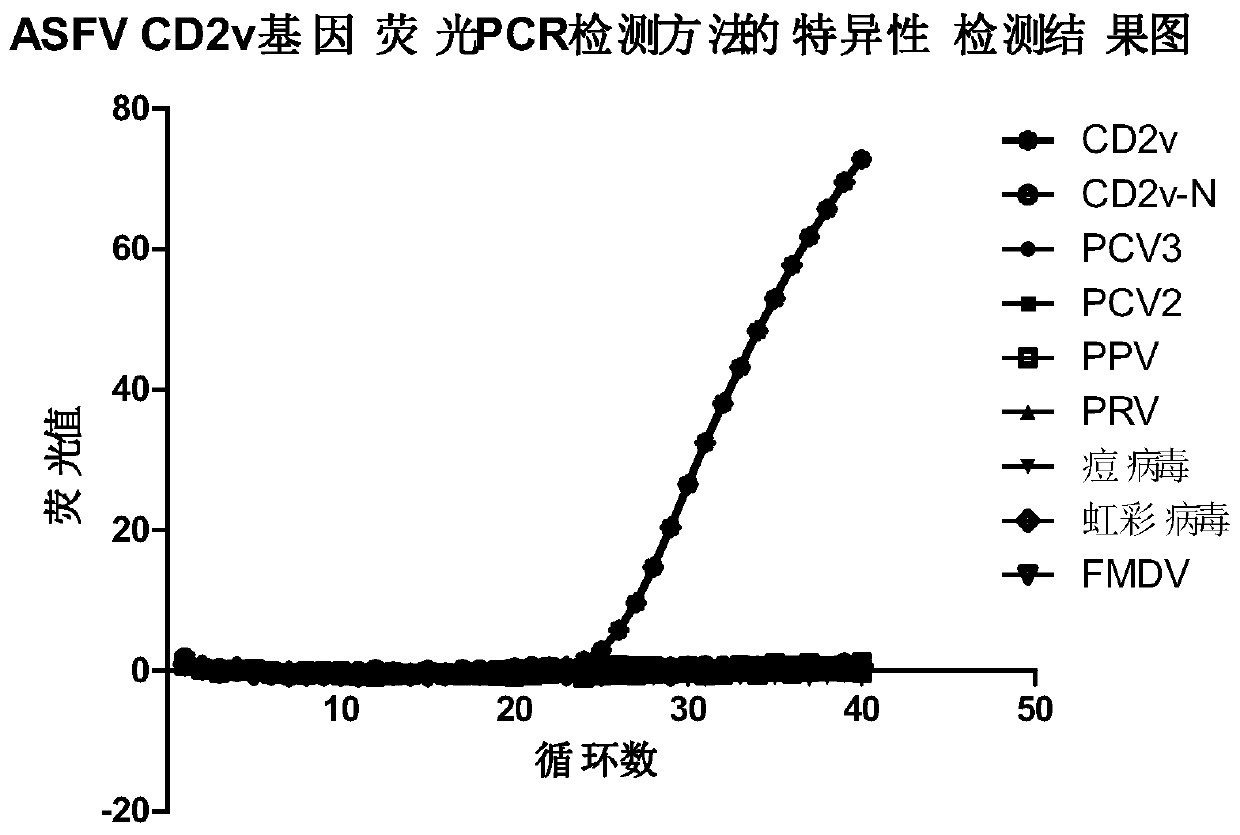
[0074] Example 1 Establishment of CD2v Gene Fluorescent Quantitative PCR Detection Method
[0075] 1. Materials and reagents: Inactivated ASFV cell culture medium was provided by the Polish National Veterinary Research Institute; DNA samples of PCV3, PCV2, porcine pseudorabies virus, porcine parvovirus, iridovirus, pox virus, and foot-and-mouth disease virus were provided by the Chinese Academy of Inspection and Quarantine Preserved by the Institute of Animal Quarantine. Wizard Genomic DNA Purification Kit was purchased from Promega, and 2×ExTaq Mix for qPCR (TaKaRa) was purchased from Beijing Liuhetong Economic and Trade Co., Ltd.
[0076] 2. Extraction of virus DNA from inactivated cell culture medium
[0077]Take 100 μl of inactivated ASFV cell culture medium to extract DNA according to the instructions of the Wizard Genomic DNA Purification Kit kit, and finally dissolve the DNA with 50 μl of DNA lysis solution, and store it at -20°C for future use.
[0078] 3. Primer Des...
Embodiment 2
[0093] Example 2 Establishment of eGFP gene fluorescence quantitative PCR detection method
[0094] 1. Materials and reagents: ASFV recombinant vaccine virulent cell culture fluid DNA was provided by the Academy of Military Medical Sciences of the Chinese People's Liberation Army; PCV3, PCV2, porcine pseudorabies virus, porcine parvovirus, iridescent virus, pox virus, and foot-and-mouth disease virus DNA samples were inspected and quarantined by China Preserved by the Institute of Animal Quarantine, Academy of Sciences. Wizard Genomic DNA Purification Kit was purchased from Promega, and 2×ExTaq Mix for qPCR (TaKaRa) was purchased from Beijing Liuhetong Economic and Trade Co., Ltd.
[0095] 2. Primer Design
[0096] According to the sequence of the eGFP gene, a set of fluorescent PCR primers and probes were designed using Beacon Design software. The specific primer sequences are as follows:
[0097] eGFPF: 5'-CAGGAGCGCACCATCTTC-3'
[0098] eGFPR: 5'-AAGTCGATGCCCTTCAGC-3'
...
Embodiment 3
[0110] Example 3 Establishment of ASFV P72 / CD2v dual fluorescent quantitative PCR detection method
[0111] 1. Materials and reagents: The inactivated ASFV cell culture medium was provided by the Polish National Veterinary Research Institute, and the nucleic acid DNA of samples 1, 2, 3, and 4 was provided by the Academy of Military Medical Sciences of the Chinese People's Liberation Army. Wizard Genomic DNA Purification Kit was purchased from Promega, and 2×ExTaq Mix for qPCR (TaKaRa) was purchased from Beijing Liuhetong Economic and Trade Co., Ltd.
[0112] 2. Extraction of DNA from inactivated ASFV cell culture fluid
[0113] Take 100 μl of inactivated ASFV cell culture medium to extract DNA according to the instructions of the Wizard Genomic DNA Purification Kit kit, and finally dissolve the DNA with 50 μl of DNA lysis solution, and store it at -20°C for future use.
[0114] 3. Establishment of dual fluorescent quantitative PCR detection method for ASFV P72 / CD2v
[0115] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com