Method for preparing antiviral drug oseltamivir phosphate intermediate tert-butylamine derivative I
A technology for antiviral drugs and tert-butylamine, which is applied in the field of preparing antiviral drug Tamiflu intermediate tert-butylamine derivatives to achieve the effects of avoiding curing and increasing by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
reference example 1
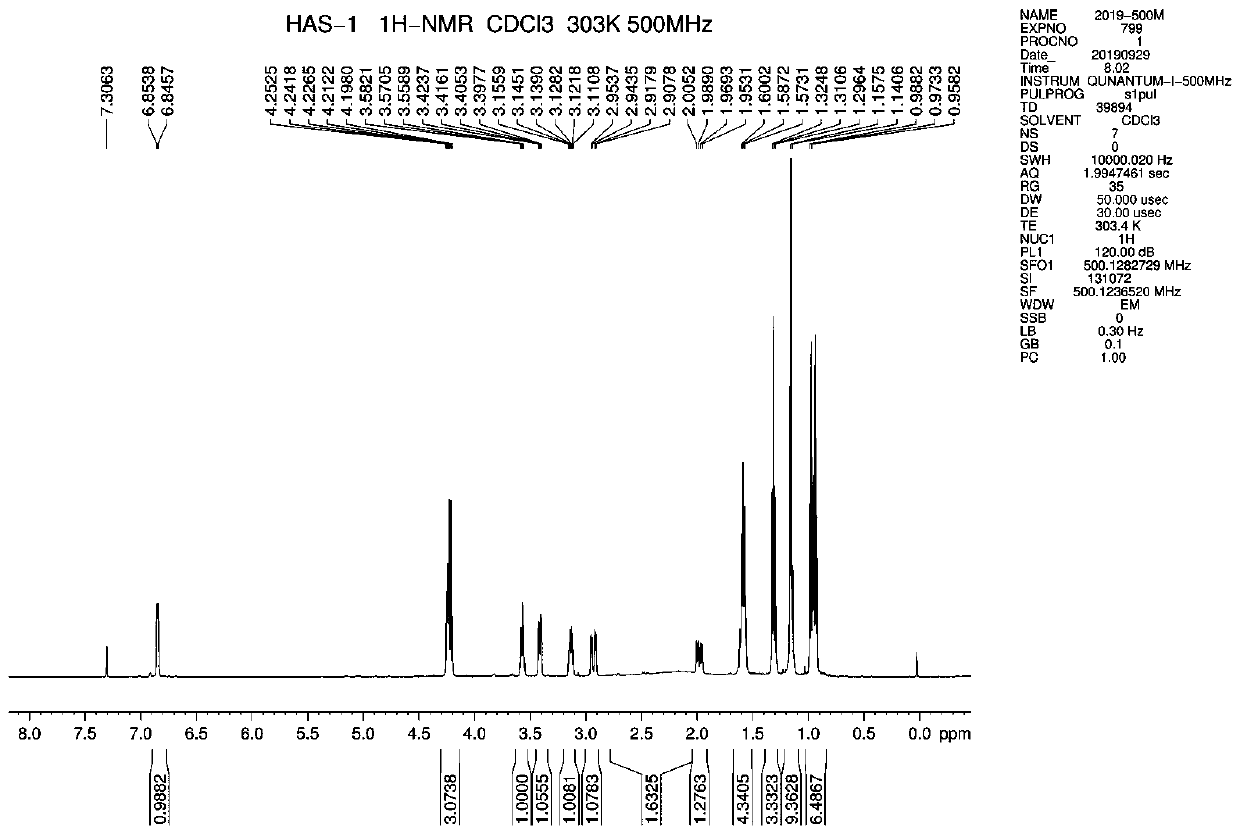
[0057] Reference Example 1: (3R, 4S, 5R)-5-(tert-butylamino)-4-hydroxyl-3-(pent-3-yloxy)cyclohex-1-ene-1-carboxylic acid ethyl ester ( 1) (conventional method for preparing compound 1 disclosed in the patent CN100545145C)
[0058] 20.22g of magnesium chloride (212.33mmol, 0.9eq), 25.88g of tert-butylamine (353.88mmol, 1.5eq) and 120ml of toluene were added and stirred for 6 hours, during which time the system solidified severely. A solution of 60.00 g of compound B (235.92 mmol) and 150 ml of toluene was added into the system at 25° C., the temperature was raised to 50° C., and the reaction was carried out for 8 hours. An additional 20.71 g of tert-butylamine (283.10 mmol, 1.2 equivalents) was added to continue the reaction for 12 hours.
[0059]Stop heating, lower the temperature to 20-25°C, slowly add 210ml of 10% citric acid dropwise, separate the liquids, and concentrate the organic phase to dryness at 45°C under reduced pressure. Brown oil compound 1 was obtained: 69.30...
reference example 2
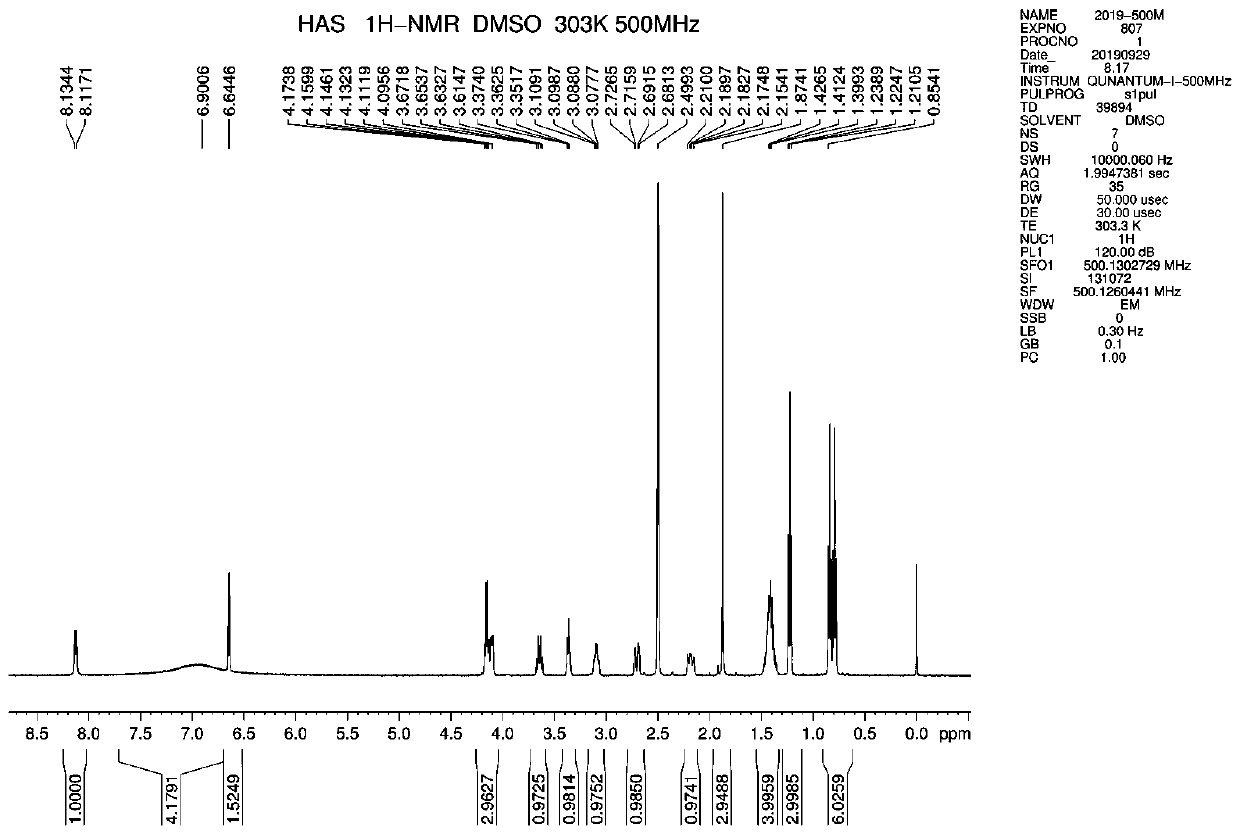
[0063] Reference Example 2: (1R, 5R, 6R)-7-(tert-butyl)-5-(pent-3-yloxy)-7-azabicyclo[4.1.0]hept-3-ene-3- Ethyl carboxylate (2)
[0064] Add 65.00g of compound 1 (198.50mmol) and 240ml of toluene, cool down to 0-5°C, replace with nitrogen, slowly add 24.33g of methanesulfonyl chloride (212.40mmol, 1.07 equivalents) dropwise, control the temperature at 5-10°C and stir for 1 hour, then cool down 40.77g of triethylamine (402.95mmol, 2.03 equivalents) was slowly added dropwise at 0-5°C, stirring was continued for 0.5 hours, and the temperature was raised to 70°C for 3 hours.
[0065] Post-treatment: lower the temperature to 10-15°C, and add dropwise an aqueous solution of potassium carbonate (27.71g of potassium carbonate dissolved in 98ml of water). The liquid was separated, and the organic phase was concentrated to dryness under reduced pressure at 45° C. to obtain 61.20 g of brown oil compound 2 with a yield of 99.70%.
reference example 3
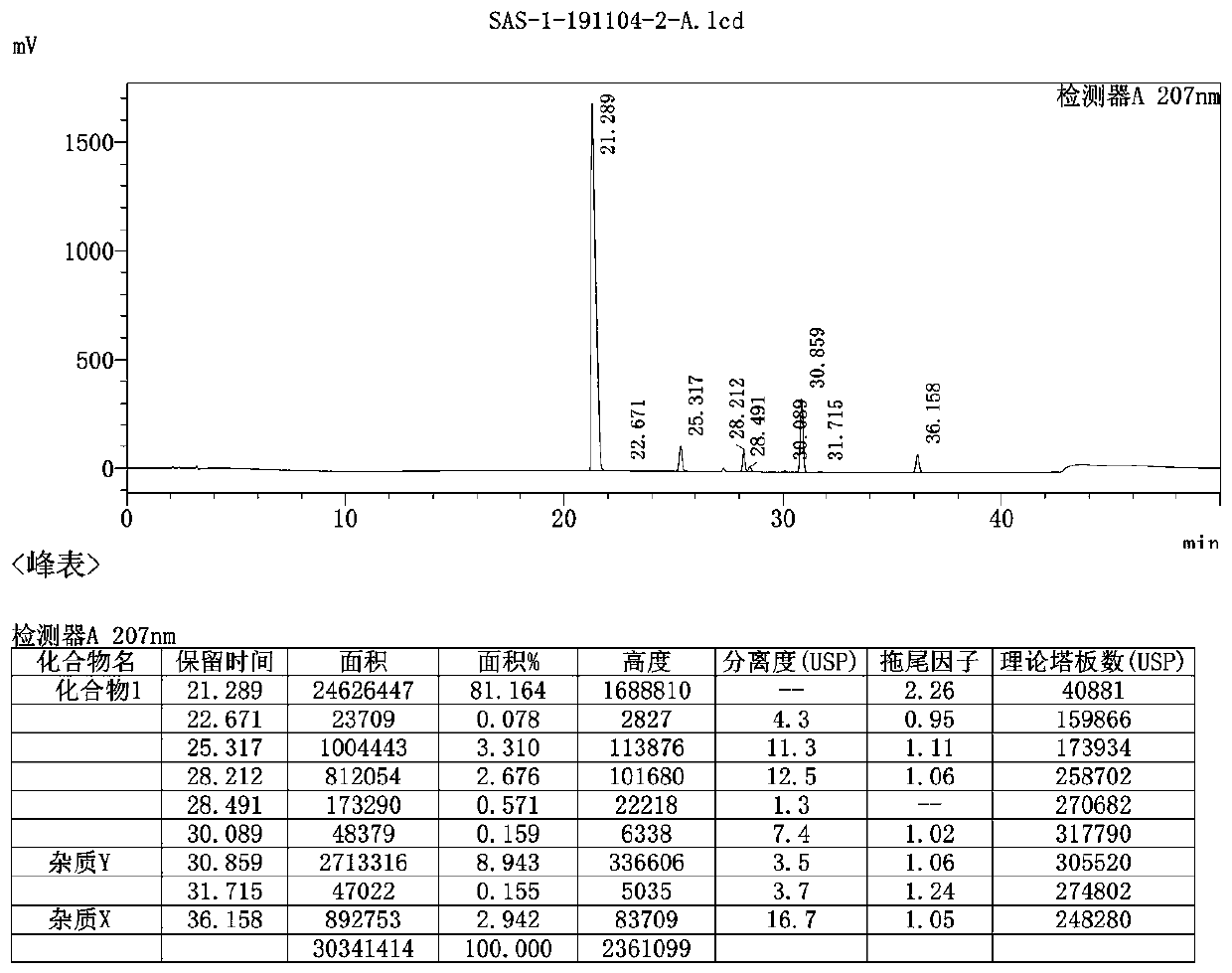
[0066] Reference Example 3: (3R, 4R, 5S)-4-(tert-butylamino)-5-(diallylamino)-3-(pent-3-yloxy)cyclohex-1-ene-1 - Ethyl carboxylate (3)
[0067] Add 55.00g of compound 2 (177.74mmol), 23.30g of diallylamine (239.95mmol, 1.35eq) and 34.86g of benzenesulfonic acid (220.40mmol, 1.24eq), raise the temperature to 120°C, and react for 5.5h.
[0068] Cool down, add 82.5ml of toluene, continue to cool down to 5-10°C, slowly add NaOH solution prepared by 8.96g of sodium hydroxide and 110ml of water, complete the addition, and separate the liquids. The organic phase was adjusted to pH=7-8 with 1N hydrochloric acid, separated, and the organic phase was concentrated to dryness at 40°C under reduced pressure to obtain 61.30 g of brown oil compound 3, yield: 84.82%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com