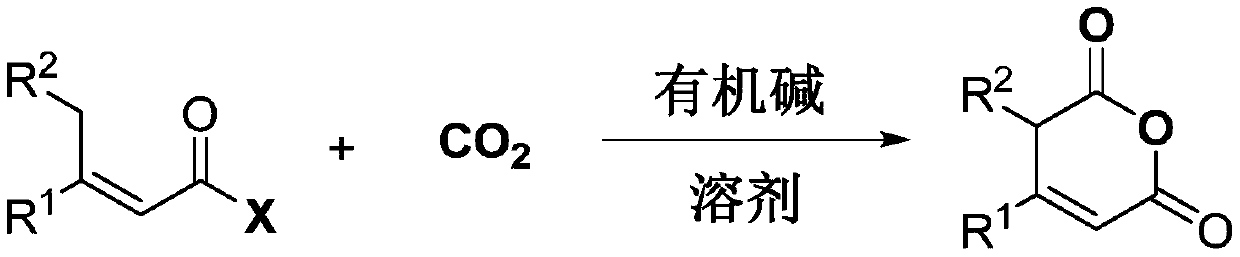
Method for synthesizing pentene dianhydride compound by using carbon dioxide
A glutacinic anhydride and carbon dioxide technology, applied in the direction of organic chemistry, can solve the problems of insufficient economic greenness, low efficiency, and long reaction steps, and achieve the effects of environmental friendliness, safe and simple operation, and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Add a stirring bar, 0.1 mmol of 3-phenyl-2-butenoic acid pentafluorophenyl ester, 0.3 mmol of 1,8-diazabicycloundec-7-ene into a 20 ml autoclave , 2 milliliters of acetonitrile, rushed into 1.0MPa carbon dioxide, after stirring at 25 degrees Celsius for 24 hours, slowly released unreacted carbon dioxide, and the reaction solution was rotary evaporated under reduced pressure to remove acetonitrile to obtain a crude product, which was purified by column chromatography to obtain the target product. The eluent used in column chromatography was ethyl acetate and acetic acid in a volume ratio of 100:1. The yield was 90%.
Embodiment 2
[0024] Add a stirring bar, 0.1 mmol of 3-phenyl-2-butenoic acid pentafluorophenyl ester, 0.10 mmol of 1,8-diazabicycloundec-7-ene into a 20 ml autoclave , 2 milliliters of acetonitrile, rushed into 1.0MPa carbon dioxide, after stirring at 25 degrees Celsius for 24 hours, slowly released unreacted carbon dioxide, and the reaction solution was rotary evaporated under reduced pressure to remove acetonitrile to obtain a crude product, which was purified by column chromatography to obtain the target product. The eluent used in column chromatography was ethyl acetate and acetic acid in a volume ratio of 100:10. The yield was 64%.
Embodiment 3
[0026] Add a stirring bar, 0.1 mmol of 3-phenyl-2-butenoic acid pentafluorophenyl ester, 0.3 mmol of 1,8-diazabicycloundec-7-ene into a 20 ml autoclave , 2 milliliters of acetonitrile, rushed into 0.1MPa carbon dioxide, stirred at 25 degrees Celsius for 24 hours, slowly released unreacted carbon dioxide, and the reaction liquid was rotary evaporated under reduced pressure to remove acetonitrile to obtain a crude product, which was purified by column chromatography to obtain the target product. The eluent used in column chromatography was ethyl acetate and acetic acid in a volume ratio of 100:1. The yield was 84%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com