Aromatic ring-containing benzophenone derivatives, and preparation method and application thereof
A technology of benzophenone and methoxybenzophenone, which is applied in the field of benzophenone derivatives and its preparation, can solve the problems of narrow absorption range, unsatisfactory activity, and weak absorption strength, etc., and achieves easy preparation , Reduce the release of benzene, good compatibility effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Add 0.228g (1mmol) of 2-hydroxy-4-methoxybenzophenone and 0.150g (1.5mmol) of triethylamine into the container, dissolve it with 3ml of dichloromethane, and slowly add 4 -Toluyl chloride 0.185g (1.2mmol), stirred for 3 hours. After the reaction, add water to the reaction solution and extract with ethyl acetate, collect the organic phase, wash with water, dry over anhydrous sodium sulfate, and remove the organic solvent by rotary evaporation; and use petroleum ether / ethyl acetate (volume ratio 12:1) as The eluent was subjected to column chromatography to obtain derivative 1.
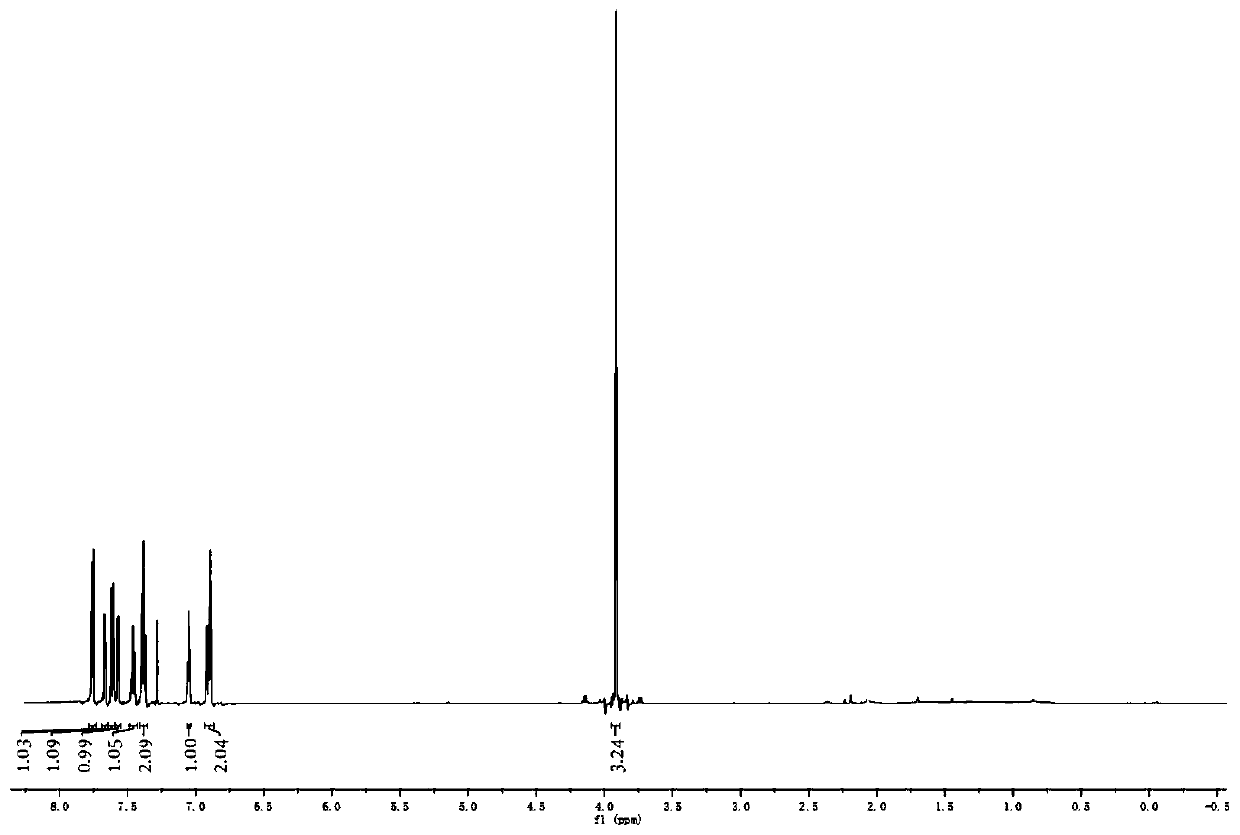
[0035] Derivative 1: colorless transparent liquid, yield: 94%, 1 H NMR (600MHz, CDCl 3 )δ7.78–7.72 (m, 4H), 7.60 (t, J = 8.0Hz, 1H), 7.45 (dt, J = 8.6, 4.1Hz, 1H), 7.37 (dd, J = 16.1, 8.3Hz, 2H ),7.16(t,J=8.7Hz,2H),6.93–6.86(m,2H),3.96–3.86(m,3H),2.43–2.36(m,3H). 13 C NMR (151MHz, CDCl 3 )δ194.30, 164.74, 163.05, 151.03, 144.35, 138.51, 132.59, 132.40, 130.10, 129.62, 129.03, 128.21, 126.12, 1...
Embodiment 2
[0037] Add 0.228g (1mmol) of 2-hydroxy-4-methoxybenzophenone and 0.150g (1.5mmol) of triethylamine into the container, dissolve it with 3ml of dichloromethane, and slowly add 2 - Thienoyl chloride 0.175g (1.2mmol), stirred for 3 hours. After the reaction, add water to the reaction solution and extract with ethyl acetate, collect the organic phase, wash with water, dry over anhydrous sodium sulfate, and remove the organic solvent by rotary evaporation; and use petroleum ether / ethyl acetate (volume ratio 12:1) as The eluent was subjected to column chromatography to obtain derivative 2.
[0038] Derivative 2: white solid, yield: 96%, m.p.86-88°C. 1 H NMR (600MHz, CDCl 3 )δ7.75(t, J=7.9Hz, 2H), 7.69–7.65(m, 1H), 7.60(dd, J=10.4, 5.5Hz, 1H), 7.58–7.55(m, 1H), 7.47(dd ,J=14.3,7.0Hz,1H),7.41–7.35(m,2H),7.04(dd,J=11.1,6.4Hz,1H),6.93–6.86(m,2H),3.94–3.88(m,3H ). 13 C NMR (151MHz, CDCl 3 )δ 194.18, 163.01, 160.00, 150.48, 138.43, 134.79, 133.67, 132.67, 132.42, 132.11, 129.61, 128...
Embodiment 3
[0040] Add 0.228g (1mmol) of 2-hydroxy-4-methoxybenzophenone and 0.150g (1.5mmol) of triethylamine into the container, dissolve it with 3ml of dichloromethane, and slowly add 2 - 0.156 g (1.2 mmol) of furoyl chloride, stirred and reacted for 3 hours. After the reaction, add water to the reaction solution and extract with ethyl acetate, collect the organic phase, wash with water, dry over anhydrous sodium sulfate, and remove the organic solvent by rotary evaporation; and use petroleum ether / ethyl acetate (volume ratio 12:1) as The eluent was subjected to column chromatography to obtain derivative 3.
[0041] Derivative 3: white solid, yield: 91%, m.p.105-108°C. 1 H NMR (600MHz, CDCl 3 )δ7.74(d, J=7.7Hz, 2H), 7.63–7.55(m, 2H), 7.47(q, J=7.3Hz, 1H), 7.39(t, J=7.6Hz, 2H), 7.05– 6.99(m,1H),6.94–6.86(m,2H),6.46(dd,J=3.3,1.5Hz,1H),3.94–3.87(m,3H). 13 C NMR (151MHz, CDCl 3 )δ194.09, 163.05, 156.27, 150.18, 147.23, 143.37, 138.41, 132.78, 132.40, 129.61, 128.20, 123.93, 119.57, 11...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com