Methods and compositions for cancer treatment
A technology of composition and immunoconjugate, which is used in the field of cancer treatment and composition, and can solve the problems of drug resistance of chemotherapeutic agents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0238] 1. A composition comprising an immunoconjugate and a cytotoxic agent, wherein: the immunoconjugate comprises 1) one or more interleukins, and 2) an interleukin consisting of a first Fc subunit and a second Fc subunit An Fc domain, the first Fc subunit is associated with the second Fc subunit to form a dimer; the one or more interleukins are fused to the Fc domain; wherein the cytotoxic agent is capable of inducing immunogenicity cell death.
[0239] 2. The composition of embodiment 1, wherein at least one of the one or more interleukins is fused to the amino terminal amino acid of the Fc domain.
[0240] 3. The composition according to any one of embodiments 1 to 2, wherein the immunoconjugate comprises two or more interleukins.
[0241] 4. The composition of embodiment 3, wherein at least two of the two or more interleukins are fused to the amino terminal amino acid of the Fc domain.
[0242] 5. The composition according to any one of embodiments 1 to 4, wherein one ...
Embodiment 1
[0351] Modification and preparation of embodiment 1 nucleic acid
[0352] 1.1 Fc modification
[0353] Amino acid modifications (e.g., amino acid substitutions) were made to the interface residues of the human IgG1 Fc domain to obtain the following group of modifications (as shown in Table 1 below), Chain A is also referred to as Fc9 or first Fc subunit in this application , chain B is also known as Fc6 or the second Fc subunit:
[0354]
[0355]
[0356] Table 1 Amino Acid Modification Groups
[0357] Subsequently, the ScFv-Fc / Fc system was used to examine the formation of heterodimeric proteins including the modified groups listed in Table 1 above, as detailed below.
[0358] First, the human immunoglobulin γ1 (IgG1) constant region amino acid sequence (P01857) was obtained from the database Uniprot to obtain the wild-type human IgG1-Fc region amino acid sequence (SEQ ID NO: 30). A polynucleotide fragment encoding wild-type human IgG1-Fc (SEQ ID NO: 31, named Fc gen...
example 2
[0385] Construction of Example 2 Recombinant Plasmid
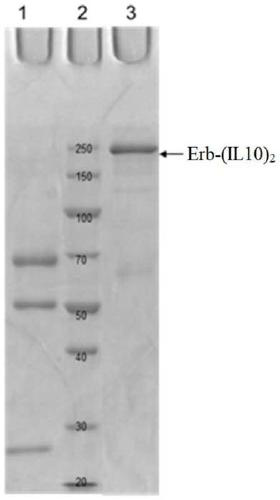
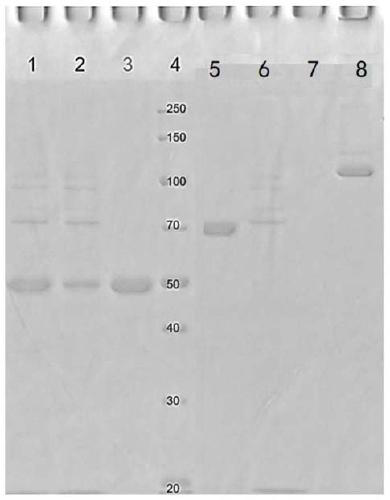
[0386] The nucleic acid molecule obtained according to Example 1 (encoding Erb-Fc9, T-Fc9, 28H1-Fc9, Fc9, T-LC (trastuzumab light chain), Erb-LC (Cetol) was digested with HindIII and EcoRI (Takara). Cyximab light chain), 28H1-LC, (IL10) 2 -Fc6 and IL10-Fc), followed by subcloning into the vector pcDNA4 / myc-HisA (Invitrogen, V863-20) respectively. The obtained plasmids were verified by sequencing, and the correct recombinant plasmids were named: pcDNA4-Erb-Fc9, pcDNA4-T-Fc9, pcDNA4-28H1-Fc9, pcDNA4-Fc9, pcDNA4-T-LC, pcDNA4-Erb-LC , pcDNA4-28H1-LC, pcDNA4-(IL10) 2 - Fc6 and pcDNA4-IL10-Fc.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com