Cobalt complex, production method therefor, and catalyst for hydrosilylation reaction
A technology of hydrosilylation and manufacturing methods, applied in the direction of organic compound/hydride/coordination complex catalysts, physical/chemical process catalysts, cobalt organic compounds, etc., can solve the problem of cobalt complexes with isonitrile ligands reported For example, there are no problems such as cobalt-isonitrile complex segregation, no catalyst utilization, etc., to achieve the effects of good solubility, easy handling, and high degree of crosslinking
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
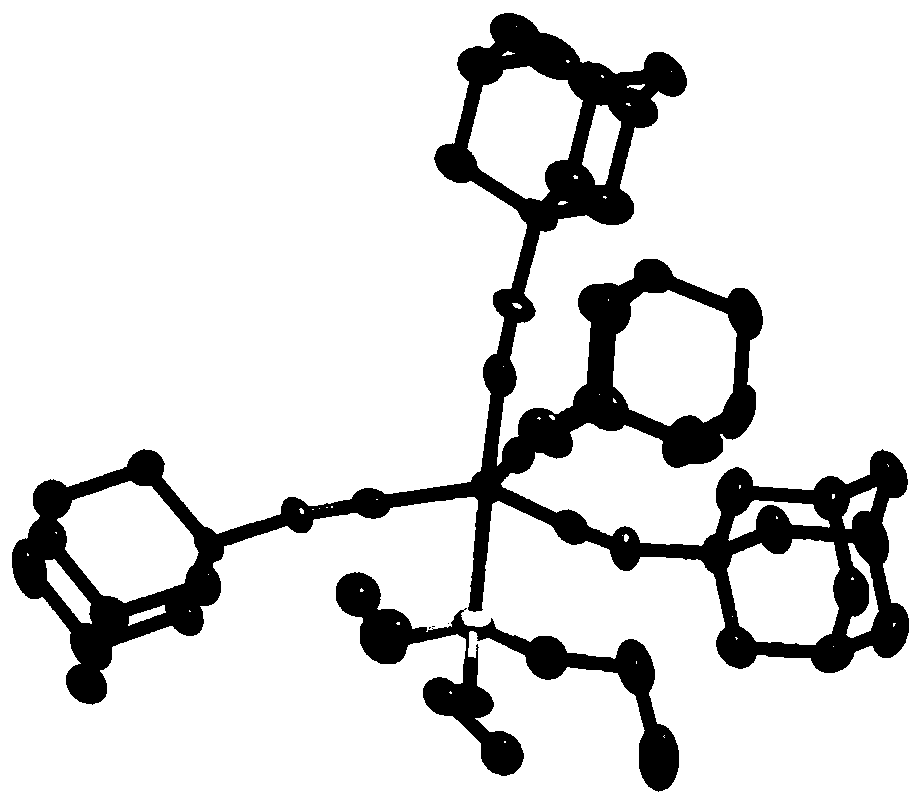
[0193] [Example 1] cobalt complex {(EtO) 3 Si}Co(CNtBu) 4 Synthesis
[0194] Add cobalt pivalate (26.2mg, 0.1mmol), benzene (100μL), tert-butylisonitrile (67.8μL, 0.6mmol) and triethoxysilane (147μL, 0.8mmol) in the reaction vessel in order, at 25 It was stirred at ℃ for 12 hours. Then, the solvent of the reaction solution and remaining triethoxysilane were distilled off under reduced pressure. The obtained dried product was dissolved in pentane (about 2 mL), cooled to -35° C., and recrystallized to obtain {(EtO) 3 Si}Co(CNtBu) 4 (38.6 mg, 70%).
[0195] Mp=150℃(Celsius)
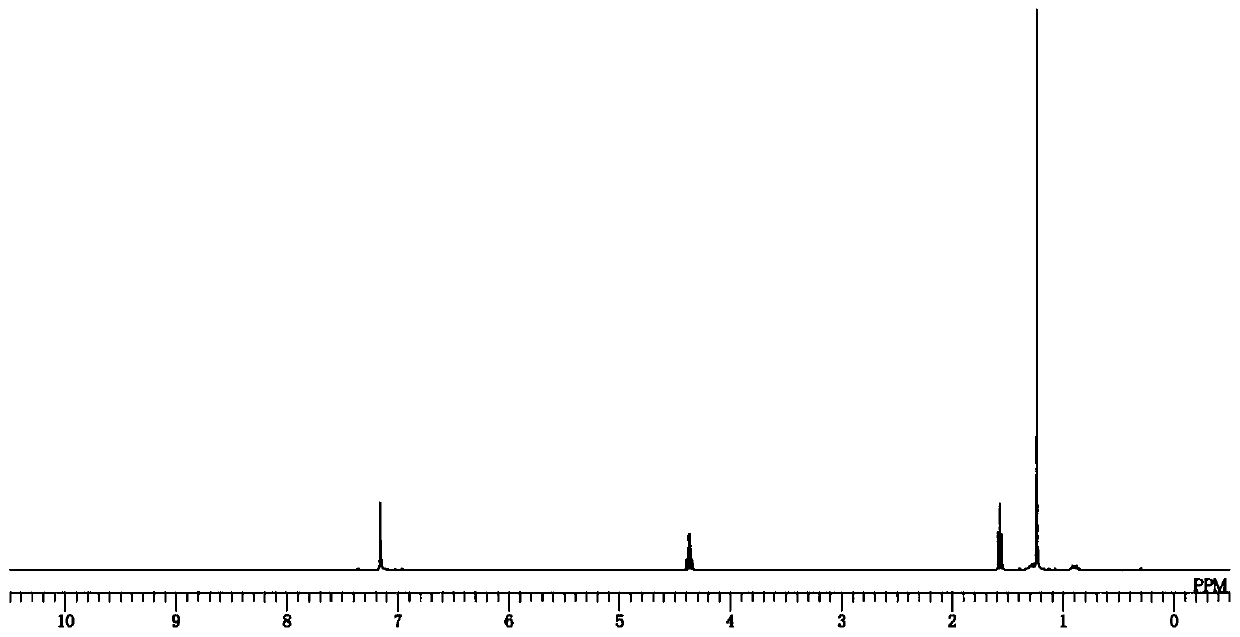
[0196] 1 H-NMR (400MHz, benzene-d 6 )δ: 4.37 (q, J=6.9, 6H), 1.57 (t, J=6.9, 9H), 1.24 (s, 36H).
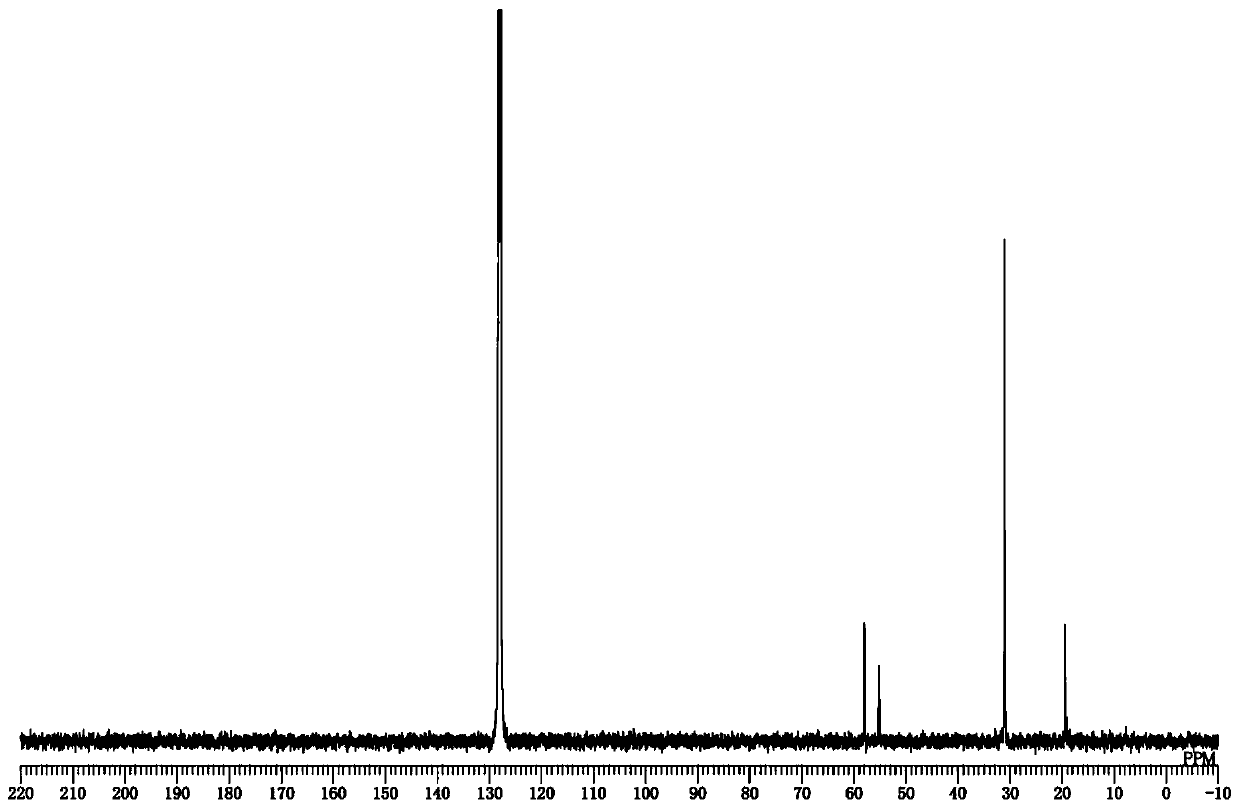
[0197] 13 C-NMR (100MHz, benzene-d 6 )δ: 58.0, 55.1, 31.1, 19.5.
[0198] 29 Si-NMR (119MHz, benzene-d 6 )δ: 0.3.
[0199] IR(ATR): νCN=2120, 2030, 2008, 1982cm -1 .
[0200] C 26 h 51 O 3 N 4 Analytical calculated value of CoSi: C56.29, H9.27, N10.10; measured value: C56.18, H9.47, N9.95. ...
Embodiment 2
[0202] [Example 2] cobalt complex {(EtO) 3 Si}Co(CNAd) 4 Synthesis of (1)
[0203] Add cobalt pivalate (26.2 mg, 0.1 mmol), benzene (100 μL), adamantyl isonitrile (96.8 mg, 0.6 mmol), and triethoxysilane (147 μL, 0.8 mmol) in the reaction vessel in order, Stirring was carried out at 25°C for 12 hours. Then, the solvent of the reaction solution and remaining triethoxysilane were distilled off under reduced pressure. The dried product obtained was dissolved in diethyl ether (about 2 mL), cooled to -35°C, and recrystallized to obtain {(EtO) 3 Si}Co(CNAd) 4 (66.8 mg, 77%).
[0204] Mp=200℃(Celsius)
[0205] 1 H-NMR (400MHz, benzene-d 6)δ: 4.49 (q, J=6.9, 6H), 2.10 (br, 24H), 1.81 (br, 12H), 1.67 (t, J=6.9, 9H), 1.40 (m, 24H).
[0206] 13 C-NMR (100MHz, benzene-d 6 )δ: 171.2, 58.2, 55.6, 44.5, 36.1, 29.6, 19.7.
[0207] 29 Si-NMR (119MHz, benzene-d 6 )δ: 0.6.
[0208] IR(ATR): νCN=2143, 2109, 1990, 1955cm -1 .
[0209] C 50 H 75 O 3 N 4 Analytical calculated va...
Embodiment 3
[0211] [Example 3] Cobalt complex {Me 2 (Me 3 SiO)Si}Co(CNtBu) 4 Synthesis
[0212] In a reaction vessel, press cobalt pivalate (26.2 mg, 0.1 mmol), benzene (100 μL), tert-butylisonitrile (67.8 μL, 0.6 mmol), 1,1,1,3,3-pentamethyldisiloxane Alkane (157 μL, 0.8 mmol) was added sequentially and stirred at 25° C. for 12 hours. Then, the solvent of the reaction solution and the remaining 1,1,1,3,3-pentamethyldisiloxane were distilled off under reduced pressure. The obtained dried product was dissolved in pentane (about 2 mL), cooled to -35°C, and recrystallized to obtain {Me 2 (Me 3 SiO)Si}Co(CNtBu) 4 (30.0 mg, 56%).
[0213] Mp=120°C (degree Celsius)
[0214] 1 H-NMR (400MHz, benzene-d 6 )δ: 1.50(s, 9H), 1.27(s, 6H), 1.20(s, 36H).
[0215] 13 C-NMR (100MHz, benzene-d 6 )δ: 170.9, 55.0, 31.1, 28.2, 8.75.
[0216] 29 Si-NMR (119MHz, benzene-d 6 )δ: 45.7, 0.2.
[0217] IR(ATR): νCN=2121, 1990, 1939 cm -1 .
[0218] C 25 H 51 O 3 N 4 CoSi 2 Analytical calculat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com