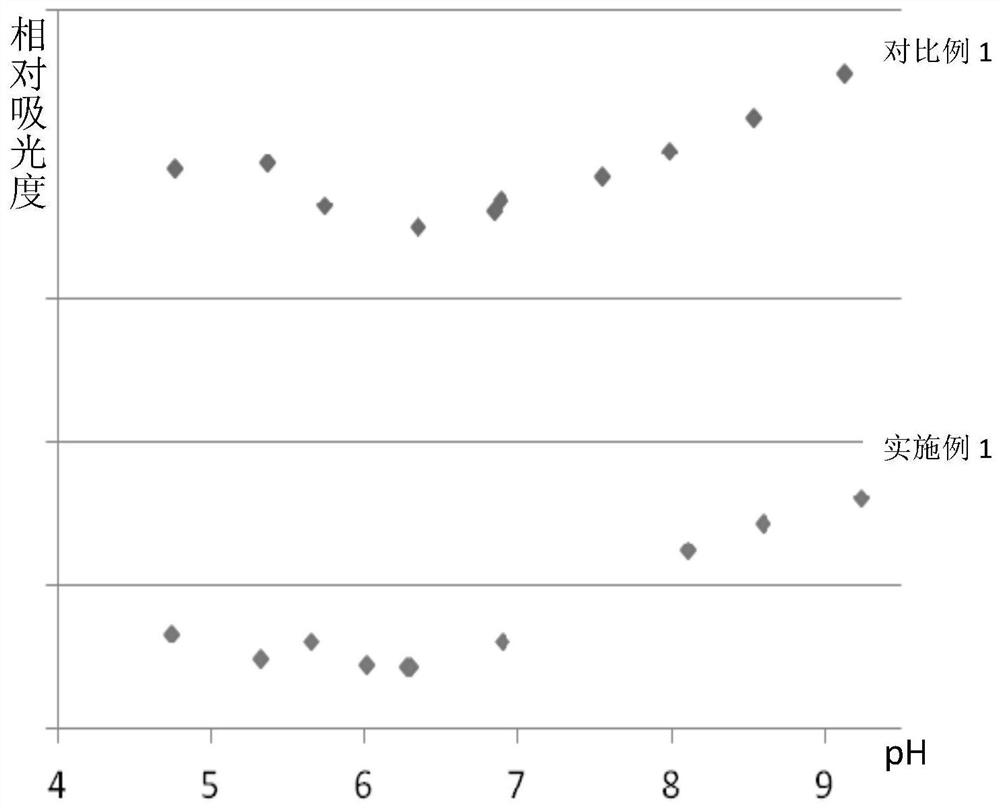
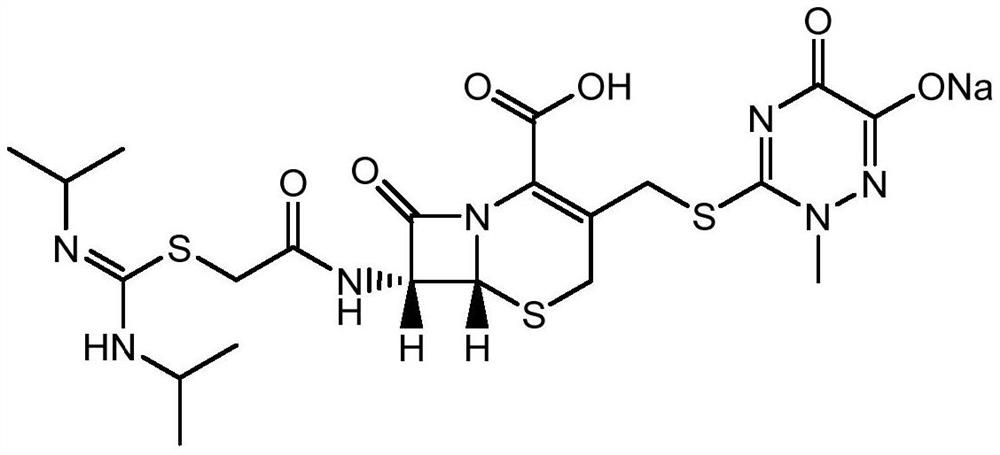
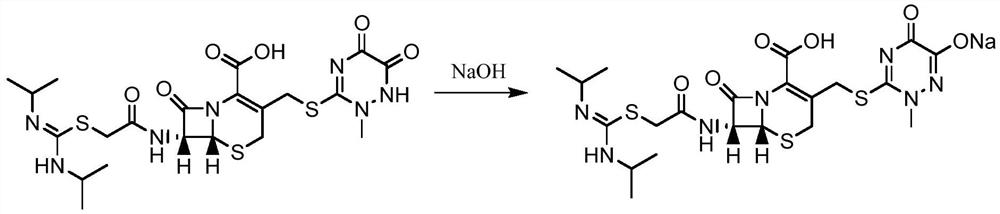
A kind of production method of cefotaxime sodium
A technology for cefotaxime sodium and a production method, which is applied to the production field of cefotaxime sodium and can solve the problems of reduced product stability, uneven reaction time, and unprominence.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0037] As an embodiment, the weight of cefotaxime in the S1: volume of organic solvent A = 1 g: (3-5) mL. The preparation method of the alkali solution is as follows: prepare an aqueous solution of sodium hydroxide or sodium carbonate, and then add the organic solvent A to dilute to form an alkali solution with a weight concentration of 5-10%. By adding the organic solvent A into the aqueous alkali solution, on the one hand, the concentration of the alkali is reduced, and on the other hand, too much water is added to prevent crystallization in the later stage. During the preparation of the alkaline solution, the ratio of the input amount of sodium hydroxide, water and organic solvent A satisfies: sodium hydroxide weight: water volume=1g: (2~3) mL, the volume of organic solvent A: sodium hydroxide aqueous solution Volume=(4~5):1.
[0038] As a preferred embodiment, when the crude product of cefotaxime sodium in S2 is refined, the crude product of cefotaxime sodium is dissolved...
Embodiment 1
[0043] The production method of the cefotaxime sodium of the present embodiment may further comprise the steps:
[0044] S1: Salt formation of cefotaxime:
[0045] S1.1: 40kg of cefotaxime and 160L of absolute ethanol were used to prepare a suspension of cefotaxime. Dissolve 2.8kg of NaOH solid in 6L of water, and then add 30L of absolute ethanol to dilute to prepare a sodium hydroxide alkali solution. Cool the solution made of 44L water and 0.1kg EDTA disodium to below 10°C, add the cefazime amidine turbid solution and sodium hydroxide alkali solution in small amounts, and keep the pH of the reaction system in the range of 5-7. , until the cefotazimemidine is completely added, continue to adjust the pH to 7.2 with sodium hydroxide solution;
[0046] S1.2: Add 4kg of activated carbon for decolorization for 15 minutes, filter, wash with 20L of 80% ethanol, and combine the washing liquid into the filtrate;
[0047] S1.3: Add 50 L of absolute ethanol dropwise. At this time, the...
Embodiment 2
[0053] The production method of the cefotaxime sodium of the present embodiment may further comprise the steps:
[0054] S1: Salt formation of cefotaxime:
[0055] S1.1: Mix 80kg of cefotaxime and 320L of absolute ethanol in batches in proportion to make a cloudy solution. Dissolve 5.6kg of NaOH solid into 12L of water and add 60L of absolute ethanol to dilute. Cool the solution made of 88L water and 200g disodium EDTA to below 10°C, add cefotaxime turbid solution and sodium hydroxide alkali solution in small amounts, and add in turns to keep the pH in the range of 5-7 until cefotaxime Add it completely, and continue to adjust the pH to 7.2 with sodium hydroxide solution;
[0056] S1.2: Add 8 kg of activated carbon to decolorize for 15 minutes, filter, wash with 30L of 80% ethanol, and combine the washing liquid into the filtrate;
[0057] S1.3: Add 100 L of absolute ethanol dropwise. At this time, the solution is slightly turbid. Add 200 g of cefotaxime sodium as a seed cr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com