Synthesis method and application of sulfo-group-containing sulfur ylide
A synthetic method, sulfur ylide technology, applied in the field of sulfur ylide synthesis, can solve problems such as unrecyclable, low economic value, difficult separation, etc., achieve the effect of reducing industrial costs, realizing solid-liquid separation, and less irritation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
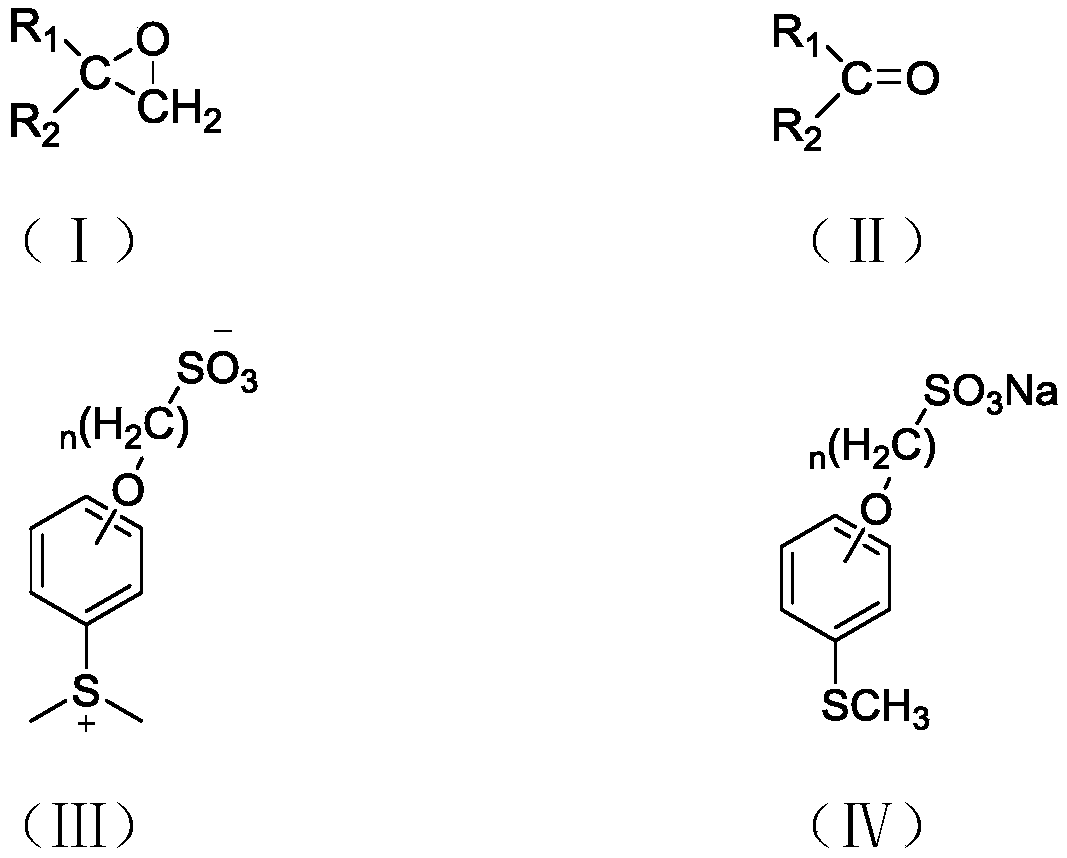
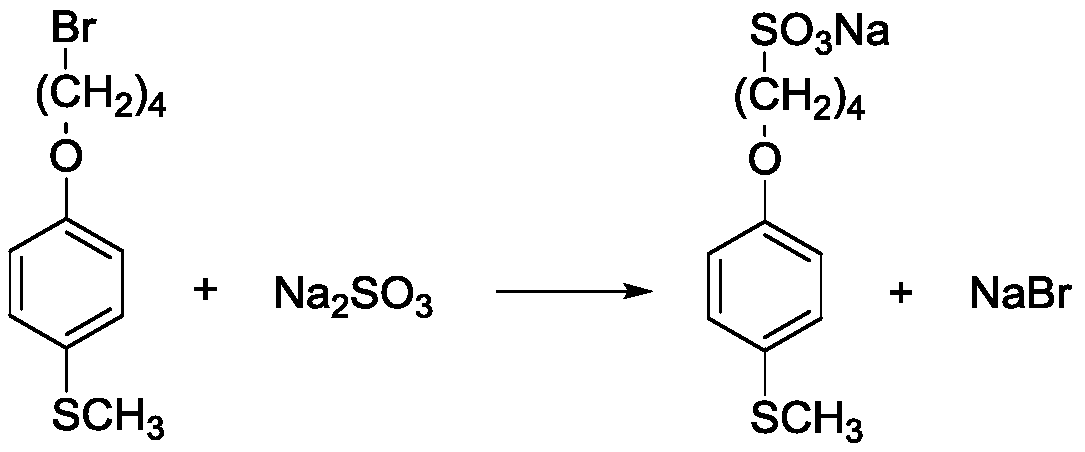
[0034] A kind of synthetic method of 4-(4-(methylthio)phenoxy)butane-1-sodium sulfonate, described method is: In a 500ml four-neck flask, add 12.6g (0.10mol) of anhydrous sodium sulfite, 28.0g (0.10mol) of 1-(4-bromobutoxy)-4-(methylthio)benzene, 120ml of distilled water, and 110ml of ethanol in sequence , 0.1g copper powder, magnetic stirring, oil bath heating to 80 ℃ reflux. After reflux for 12 h, the reaction solution was distilled off under reduced pressure to remove water until all white solids were precipitated. The resulting solid and 500ml of ethanol were added to a 1L flask, and heated to reflux for 1h. Then it was suction filtered while it was hot, and after the filtrate was cooled, it was placed overnight at 0°C. Finally, it was filtered and dried by suction to obtain sodium 4-(4-(methylthio)phenoxy)butane-1-sulfonate as a white powder crystal, with a yield of 25.1 g and a yield of 84.2%.
[0035] A kind of synthetic method of sulfur ylide containing sulfonic ac...
Embodiment 2
[0039] A kind of synthetic method of 4-(4-(methylthio)phenoxy)butane-1-sodium sulfonate, described method is: in 500ml four-necked flask, add 16.4g (0.13mol) anhydrous successively Sodium sulfite, 28.0g (0.10mol) 1-(4-bromobutoxy)-4-(methylthio)benzene, 120ml distilled water, 110ml ethanol, 0.1g copper powder, magnetic stirring, heating the oil bath to 80°C and reflux . After 15 h of reflux, the reaction solution was distilled off under reduced pressure to remove water until all white solids were precipitated. The resulting solid and 500ml of ethanol were added to a 1L flask, and heated to reflux for 1h. Then it was suction filtered while it was hot, and after the filtrate was cooled, it was placed overnight at 0°C. Finally, it was filtered and dried by suction to obtain sodium 4-(4-(methylthio)phenoxy)butane-1-sulfonate as a white powder crystal, with a yield of 25.2 g and a yield of 84.6%.
[0040]A kind of synthetic method of sulfur ylide containing sulfonic acid group, ...
Embodiment 3
[0043] A kind of synthetic method of 4-(4-(methylthio)phenoxy)butane-1-sodium sulfonate, described method is: in 500ml four-necked flask, add 20.2g (0.16mol) anhydrous successively Sodium sulfite, 28.0g (0.1mol) 1-(4-bromobutoxy)-4-(methylthio)benzene, 120ml distilled water, 110ml ethanol, 0.1g copper powder, magnetic stirring, heating the oil bath to 60°C and reflux . After 15 h of reflux, the reaction solution was distilled off under reduced pressure to remove water until all white solids were precipitated. The resulting solid and 500ml of ethanol were added to a 1L flask, and heated to reflux for 1h. Then it was suction filtered while it was hot, and after the filtrate was cooled, it was placed overnight at 0°C. Finally, it was filtered and dried by suction to obtain sodium 4-(4-(methylthio)phenoxy)butane-1-sulfonate as a white powder crystal, with a yield of 25.0 g and a yield of 84.0%.
[0044] A kind of synthetic method of sulfur ylide containing sulfonic acid group, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com