Recombinant protein G3P20-31, and preparation method and application thereof
A technology of recombinant protein and coat protein, which is applied in the field of DNA recombination, can solve the problems of cumbersome protein preparation and purification, high price, and low sensitivity, and achieve the effects of high sensitivity, low cost, and simple preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
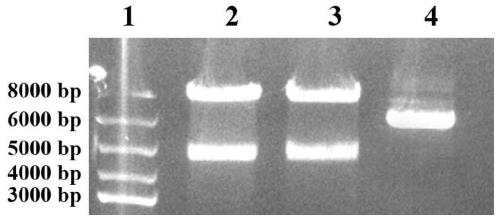
[0036] The construction of embodiment 1 recombinant phage
[0037] (1) Extraction of phage vector fADL-le
[0038] The commercialized phage vector fADL-le (purchased from Antibody Design laboratories, Catalog number: PD020) of the plasmid was extracted using the plasmid mini-extraction kit of Axygen, and the specific steps were as follows:
[0039] 1) Take 6ml of the bacterial liquid JM109 cultured overnight in LB medium (preserved in our laboratory, which contains the carrier fADL-le), centrifuge at 12000×g for 1min, and discard the supernatant;
[0040] 2) Add 250μl Buffer S1 to suspend the bacterial pellet, the suspension should be even, and no small bacterial lumps should be left;
[0041] 3) Add 250μl Buffer S2, gently and fully turn up and down 4-6 times, mix evenly, so that the bacteria are fully lysed until a clear solution is formed;
[0042] 4) Add 350μl Buffer S3, gently and fully turn up and down 6-8 times, centrifuge at 12000×g for 10min;
[0043] 5) Aspirate t...
Embodiment 2
[0089] Example 2 Preparation of phage displaying recombinant protein G3P20-31
[0090] 1) Inoculate 200 μl sequenced correct JM109 into 100 ml LB liquid medium (100 μg / ml Kar + ) in a test tube, shake vigorously at 37°C for 10 hours;
[0091] 2) 8000rpm, 10min, 4°C, keep the supernatant;
[0092] 4) Add 1 / 6 volume of PEG / NaCl solution, vortex, 4°C overnight;
[0093] 5) Centrifuge at 12000rpm for 15min, and dissolve the phage pellet with 1ml TBS;
[0094] 6) Transfer the solution to a 1.5ml EP tube, centrifuge at 14000rpm for 1min, carefully transfer the supernatant to a 1.5ml EP tube, add 150μl PEG / NaCl to each EP tube, mix well and overnight at 4°C;
[0095] 7) Centrifuge at 14,000 rpm for 15 minutes, dissolve the phage pellet with 100 μl TBS, and store in a refrigerator at 4°C.
Embodiment 3
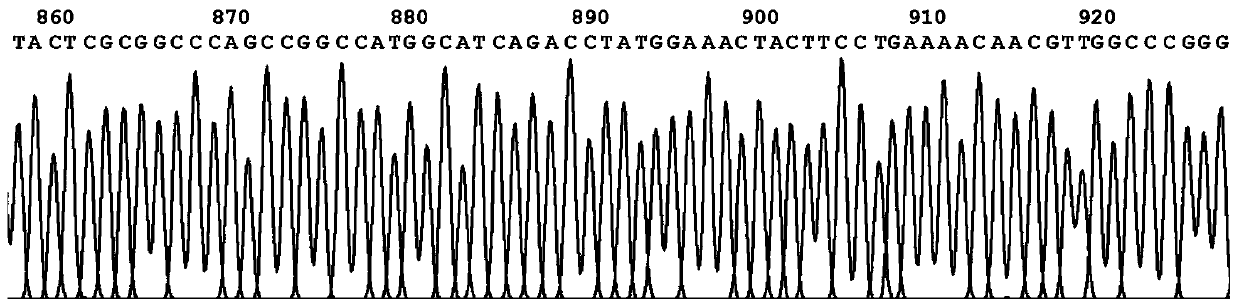
[0096] Embodiment 3Western-blot analysis shows recombinant protein G3P20-31 phage
[0097] In order to verify that the N-terminal 20-31 polypeptide of the P53 protein is fused with PIII and successfully displayed on the surface of the phage, the prepared phage were hybridized with the anti-PIII monoclonal antibody and the serum of a tumor patient with positive P53 antibody. The specific steps are as follows:
[0098] 1) After SDS-PAGE, cut off the separating gel and place it in transfer buffer;
[0099] 2) Cut the PVDF membrane and filter paper according to the size of the glue, soak the filter paper in the transfer buffer solution, soak the PVDF membrane in methanol for 1 min and then place it in the transfer buffer solution;
[0100] 3) 80V constant voltage power transfer for 2h;
[0101] 4) After ponceau staining, cut the membrane into thin strips to separate the proteins on the membrane from each other;
[0102] 5) Put the membrane into the blocking solution to seal for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com