Device and method for promoting rapid strut coverage and vascular endothelial coverage
A vascular endothelium, strut technology, applied in the field of devices that promote rapid strut coverage and vascular endothelial coverage, can solve the problems of prolonged DAPT duration, clinical and financial negative effects, and increased stent thrombosis rates.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0048] The embodiments herein and their various features and advantageous details are explained more fully with reference to the non-limiting embodiments shown in the drawings and explained in detail in the following description. Descriptions of known components and processing techniques are omitted so as not to unnecessarily obscure the embodiments herein. The examples used herein are intended merely to aid in understanding the manner in which the embodiments herein may be practiced and to further enable those skilled in the art to practice the embodiments herein. Accordingly, these examples should not be construed as limiting the scope of the embodiments herein.
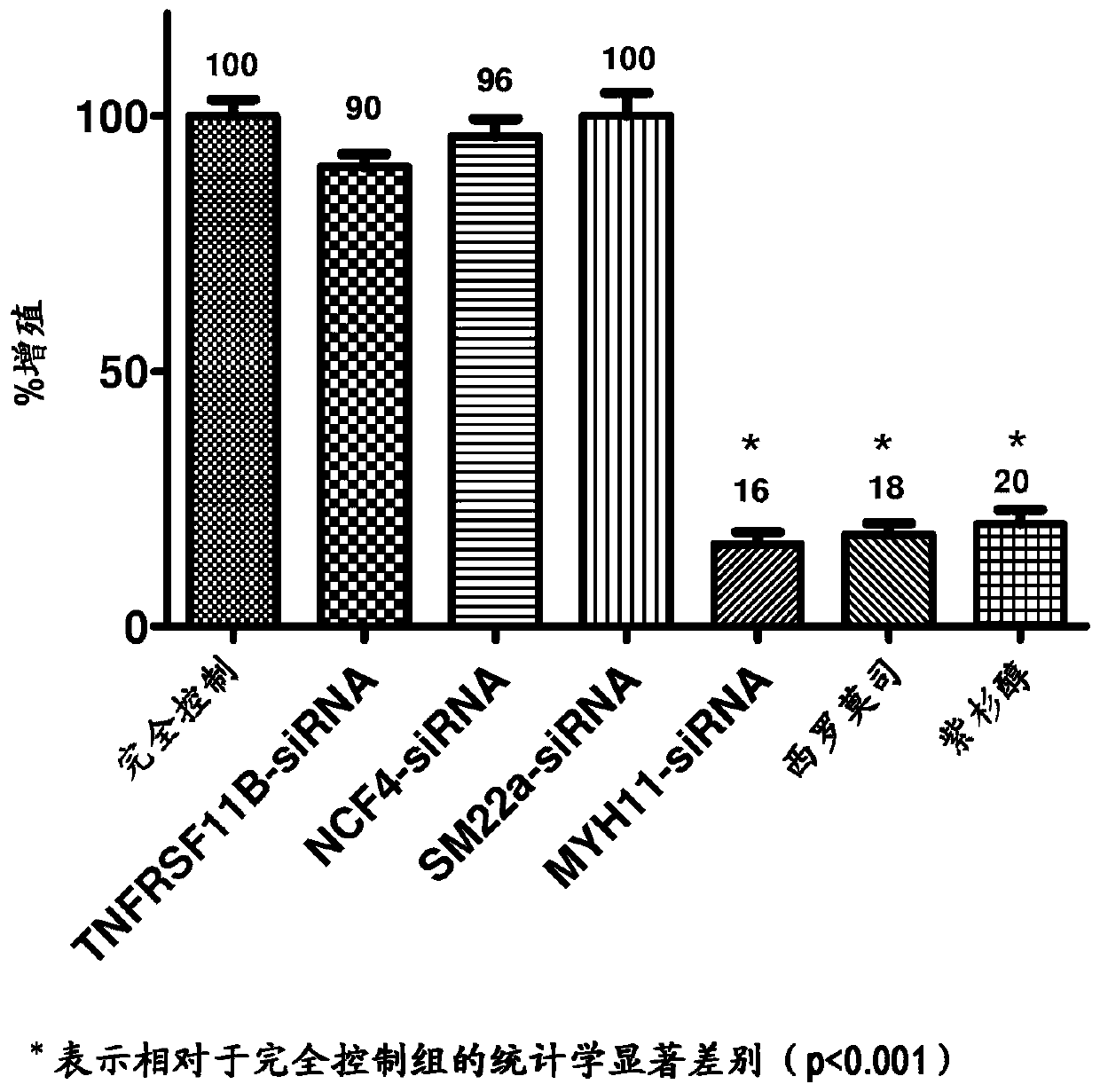
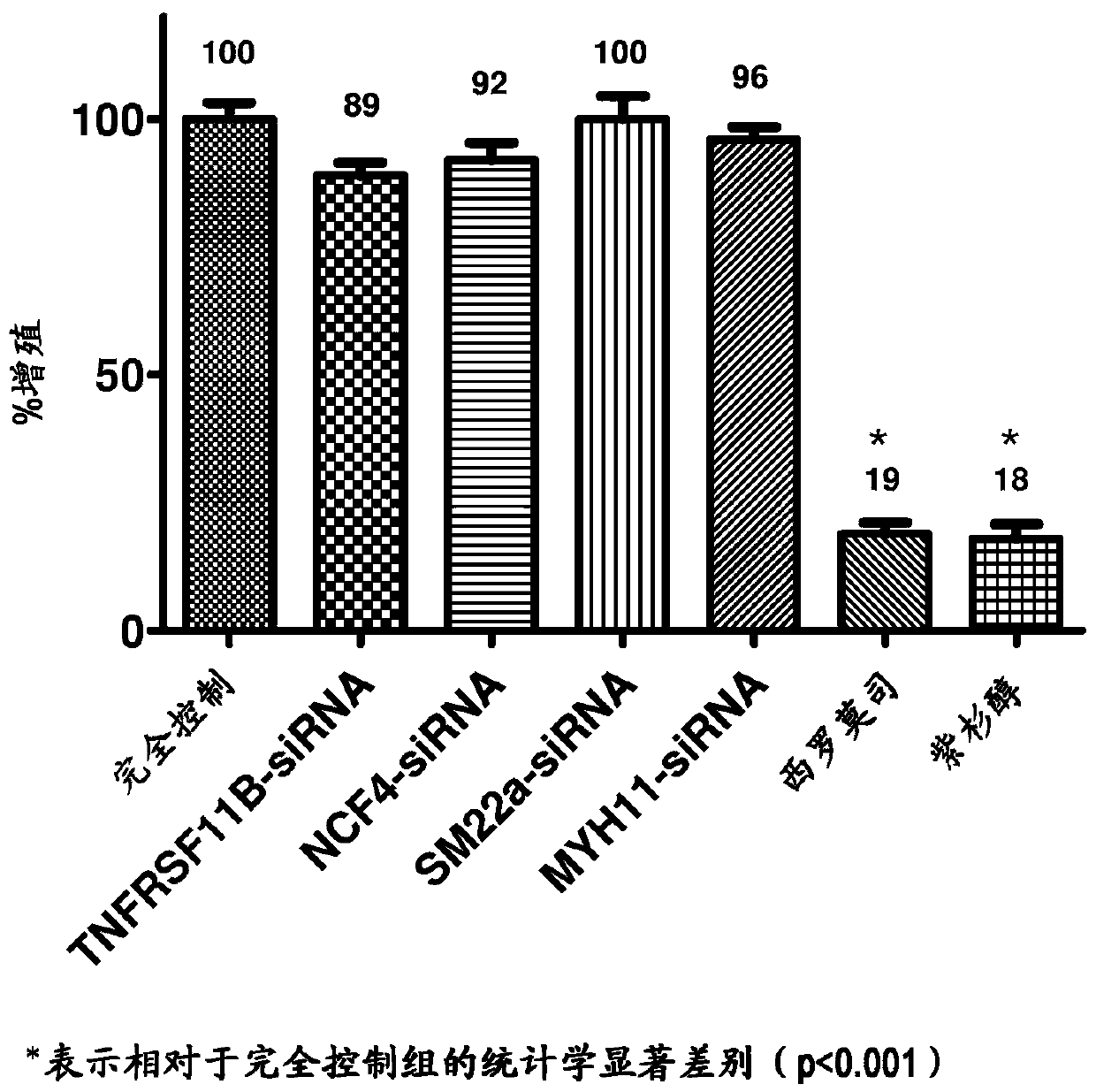
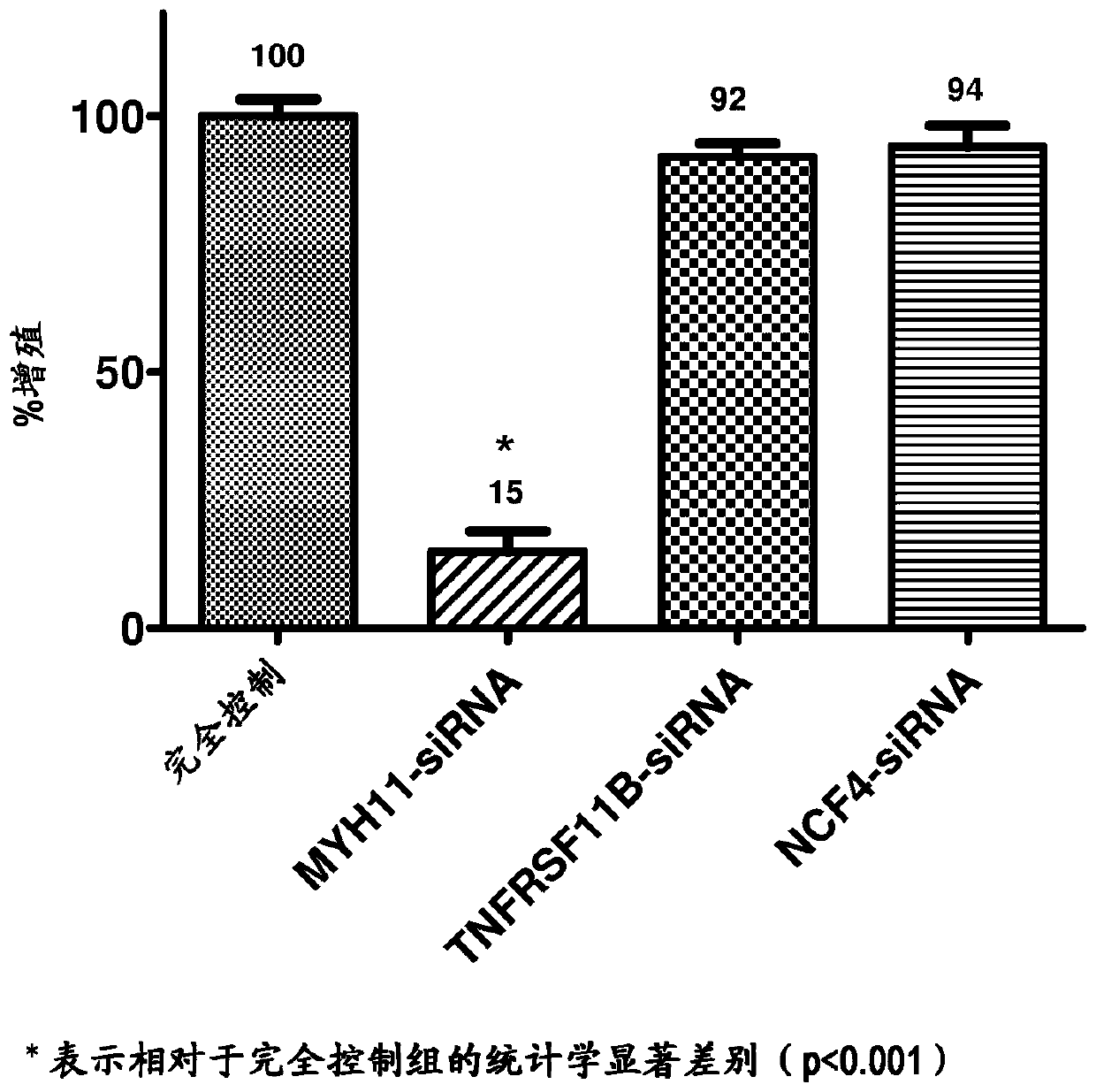
[0049] Embodiments herein are achieved by providing devices and methods for inhibiting VSMC hyperproliferation and promoting early re-endothelialization of blood vessels. The development of a medical device capable of both permanent patency and early re-endothelialization of the treated vessel segment has been pur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com