Conjugate of VEGF-GRAB protein and drug, and use thereof
A technology of conjugates and drugs, applied in 3 fields, can solve the problems of not having the function of targeting tumor cells, not showing the anti-cancer effect of cancer cells, harmful to normal blood vessels, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0109] Example 1: Cell lines and cell culture
[0110] Freestyle 293F cells (R790-07, ), A431 cells (human cervical epidermoid carcinoma, #21555, Korean Cell Line Bank), SKBR3 cells (human breast cancer, #30030, Korean Cell Line Bank), SKOV3 cells (human ovarian adenocarcinoma, #30077, ATCC) and Human umbilical vein endothelial cells (HUVEC, CC-2519, Lonza) were certified according to ATCC guidelines and used within 6 months of receipt. at 37°C and 8% CO 2 Next, Freestyle 293F cells (R790-07, ) maintained in Freestyle293F medium (12338018, ) in suspension culture with 125rpm stirring. A431 cells were cultured in DMEM (LM001-05, Welgene) supplemented with 10% heat-inactivated FBS (S001-01, Welgene) and 100 μg / ml penicillin / streptomycin, and SKBR3 cells and SKOV3 cells were supplemented with 10% heat-inactivated FBS (S001-01, Welgene) and 100 μg / ml of penicillin / streptomycin RPMI1640 (LM011-05, Welgene) were cultured, and HUVECs were incubated in gelatin (G9391, Sigma-...
Embodiment 2
[0111] Example 2: Antibodies
[0112] Antibodies useful in the present invention are shown in Table 1 below.
[0113] [Table 1]
[0114]
Embodiment 3
[0115] Embodiment 3: Expression and purification of recombinant protein
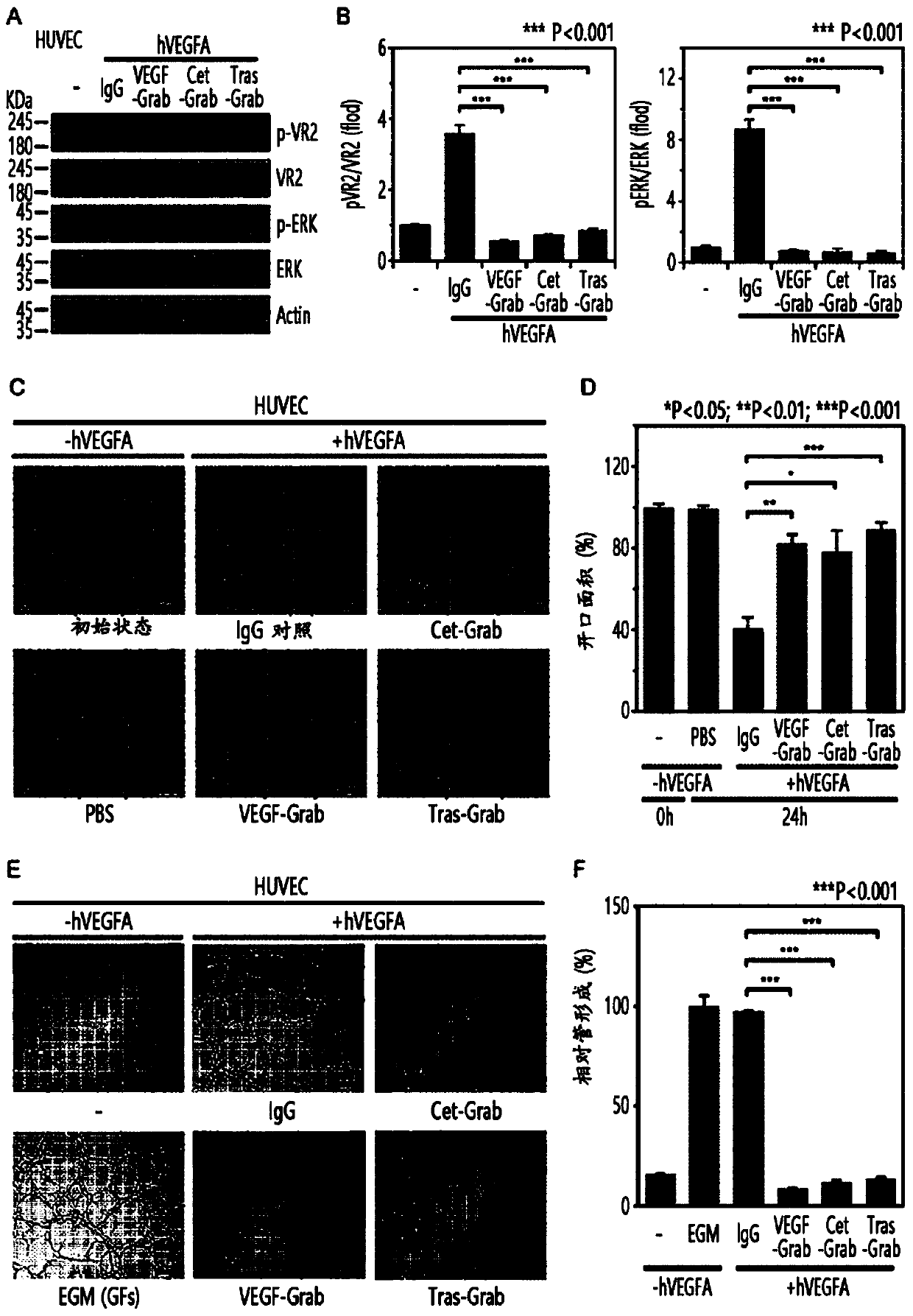
[0116] The gene encoding the cetuximab or trastuzumab single-chain variable fragment (scFv) (where the variable regions of the cetuximab or trastuzumab heavy and light chains pass through the (G4S)3 linker Connection (Ahmad ZA, Clin Dev Immunol.2012, 2012:980250.)) and VEGF-Grab's N-terminal connection (Lee JE, Mol Cancer Ther., 2015, 14:470-9) (attended figure 1 A). Polyvinylamine (765090, Sigma-Aldrich) containing VEGF-Grab, scFv-cetuximab-VEGF-Grab (Cet-Grab) and scFv-trastuzumab-VEGF-Grab (Tras-Grab) The vector was transfected into Freestyle293F cells. Transfected cells were cultured with 5 mM sodium butyrate (303410, Sigma-Aldrich) for 3 days, and then centrifuged using a centrifuge to separate only the supernatant. Supernatants containing VEGF-Grab, Cet-Grab, or Tras-Grab were purified using Protein A Sepharose (GE Healthcare LifeSciences). VEGF-Grab, Cet-Grab, or Tras-Grab were eluted with 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com