Recombinant antibody of anti-human cardiac troponin I
A troponin and antibody technology, applied in the field of immunity, can solve the problems of low activity and poor affinity, and achieve the effect of high affinity and strong binding protein activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0113] This example provides an exemplary preparation method of a recombinant antibody against human cardiac troponin I.
[0114] S10, construction of expression plasmids:
[0115] Wherein, restriction endonuclease and Prime Star DNA polymerase were purchased from Takara Company in this embodiment;
[0116] MagExtractor-RNA extraction kit was purchased from TOYOBO; BD SMART TM The RACE cDNA Amplification Kit was purchased from Takara;
[0117] The pMD-18T vector was purchased from Takara;
[0118] The plasmid extraction kit was purchased from Tiangen Company;
[0119] Primer synthesis and gene sequencing were completed by Invitrogen;
[0120] S11, design and synthesis of primers:
[0121] 5' RACE upstream primers for amplification of heavy and light chains:
[0122] Amplify Heavy Chain and Light Chain 5'RACE Primers:
[0123] SMARTER II A Oligonucleotide:
[0124] 5'>AAGCAGTGGTATCAACGCAGAGGTACXXXXX<3';
[0125] 5'-RACE CDS Primer (5'-CDS):
[0126] 5’>(T) 25 VN<3'(N...
Embodiment 2
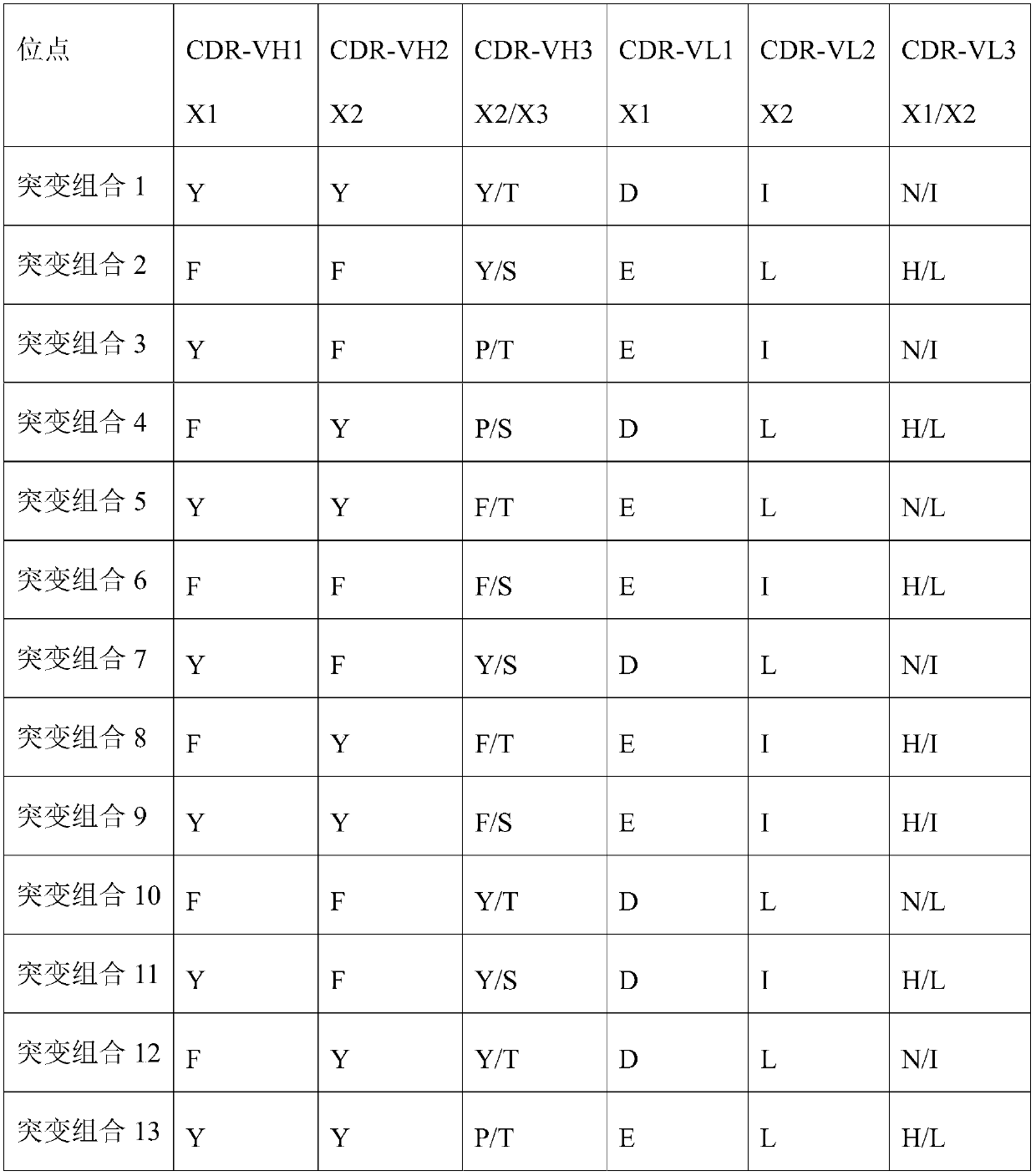
[0152] The antibody obtained in Example 1 (with sequences such as light chain and heavy chain shown in SEQ ID NO: 11 and 12) was analyzed, and the complementarity determining region (WT) of the heavy chain:
[0153] CDR-VH1 is G-F-T-Y(X1)-S-N-Y-A-M-S;
[0154] CDR-VH2 is I-S-T(X1)-G-G-S-Y-S-Y(X2)-Y-P-D-S-V-K;
[0155] CDR-VH3 is A-R-K(X1)-D-N-Y-Y(X2)-G-S-T(X3)-F-M-D-Y;
[0156] Complementarity-determining regions of the light chain:
[0157] CDR-VL1 is R-A-S-Q-D(X1)-I-T-Q(X2)-Y-L-N;
[0158] CDR-VL2 is I-Y-Y-S(X1)-S-R-I(X2)-H-S-G-V-S;
[0159] CDR-VL3 is Q-Q-G-N(X1)-T-I(X2)-P-L-T;
[0160] Among them, X1, X2, and X3 are mutation sites.
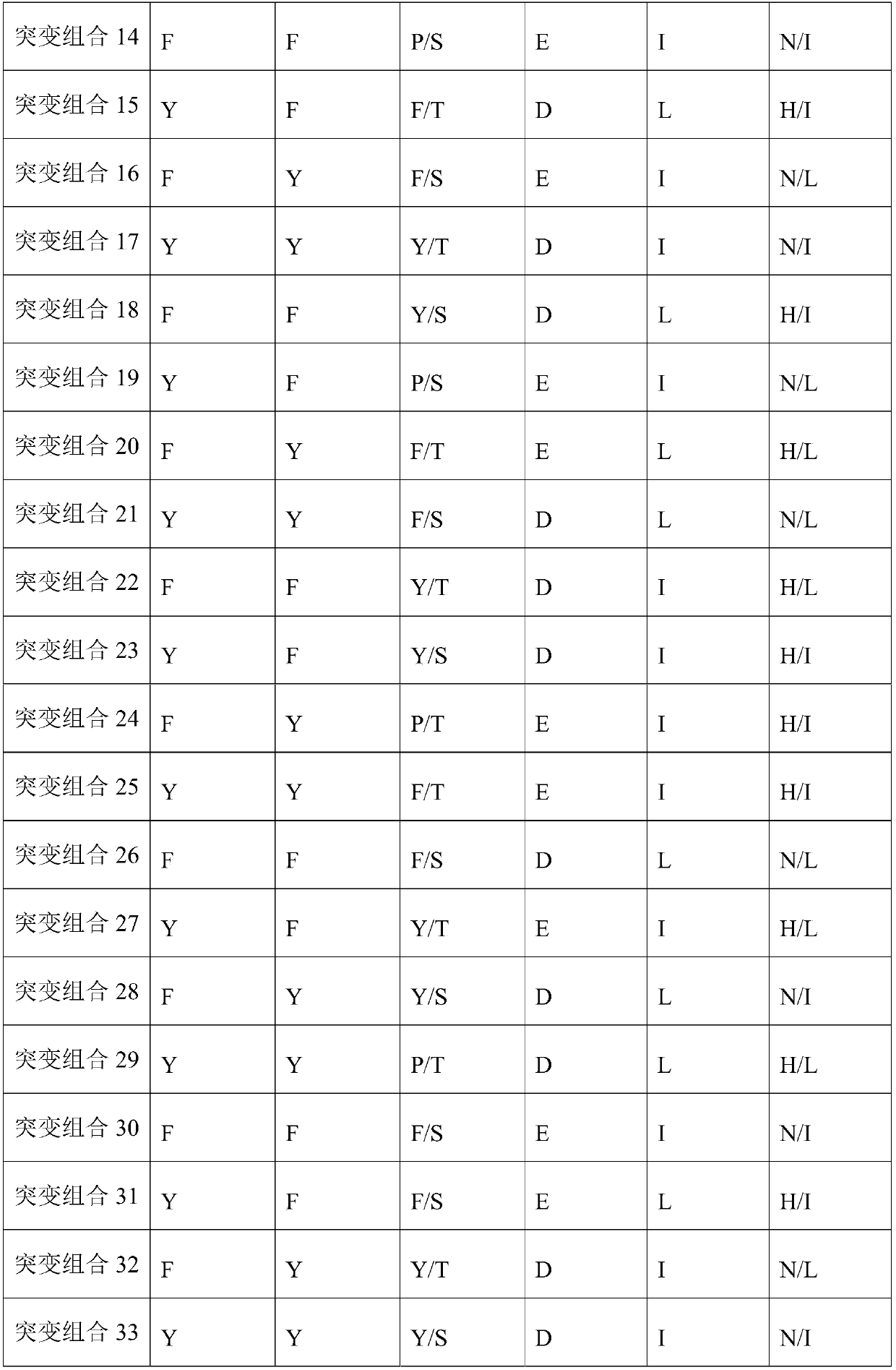
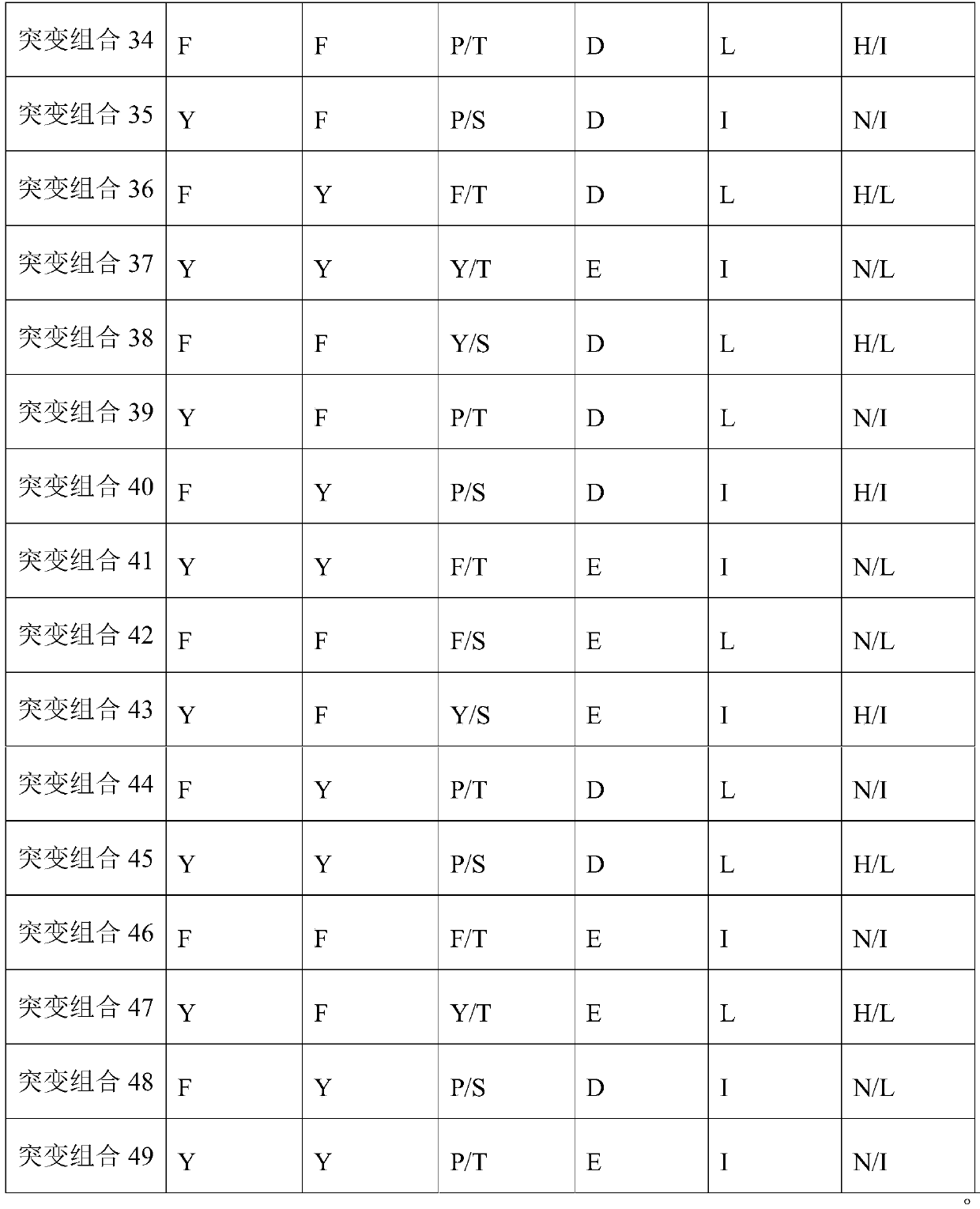
[0161] Table 1 Mutation sites related to antibody activity
[0162]
[0163] The inventors mutated the CDR sites in WT to obtain antibodies with better activity.
[0164] Dilute the CTNI quality control recombinant antigen to 1ug / ml in the coating solution to coat the microplate, 100uL per well, overnight at 4°C; the next day, wash t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com