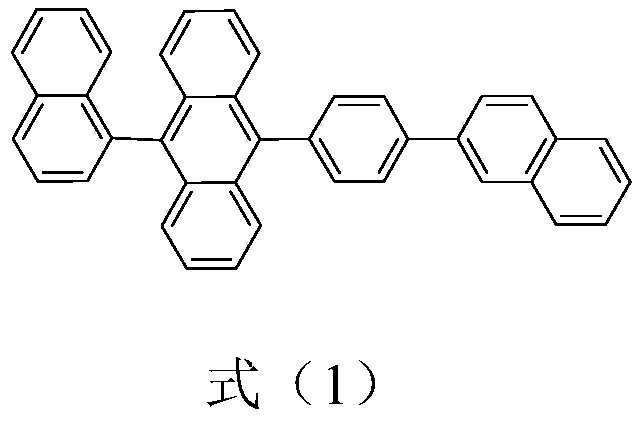
Preparation method and purification method of 9,10-substituted anthracene
A catalyst and compound technology, which is applied in the field of preparation and purification of 9--10-phenyl)anthracene, can solve the problems of low product content and large impurities, and achieves high catalytic activity, few reaction impurities and fast main reaction speed. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] A preparation method of anthracene substituted at 9,10 positions, specifically comprising the following steps:
[0046] The preparation of step 1, (2-(4-bromophenyl)naphthalene)
[0047]
[0048] Under argon protection, in a 300mL three-neck flask connected with a mechanical stirrer, a condenser, and a thermometer, add 2-naphthylboronic acid (30.0g), p-bromoiodobenzene (49.35g), Pd(PPh3) (4.60g), Potassium carbonate (24.11g), dioxane (150ml), water (60ml), reflux at 84-88°C for 9h, cool down to room temperature, filter, wash the filter cake with water until neutral (pH=7 ), after adding the reflux of 250ml toluene in the filter cake to divide water, cross the insulation column, cross the column liquid recrystallization, dry, obtain light yellow solid 2-(4-bromophenyl) naphthalene (being intermediate 1) 45.0g, The yield is 91.11%; LC-MS=283;
[0049] The preparation of step 2, 9-(1-naphthyl-) anthracene
[0050]
[0051] Under the protection of argon, into a 1L ...
Embodiment 2
[0066] The difference from Example 1 is that step 5 and the purification method in the preparation process are different, specifically:
[0067] Under the protection of argon, into a 500mL three-necked flask equipped with a mechanical stirrer, a condenser, and a thermometer, sequentially add (10-(1-naphthyl)anthracene-9-)boronic acid (10.0g), 2-(4-bromo Phenyl) naphthalene anthracene (18.31g), toluene (300ml), Pd-132 (0.020g), continue to heat up to 70 ° C ~ 76 ° C, continue to drop K 3 PO 4 Aqueous solution (K 3 PO 4 The aqueous solution consists of 58.28g K 3 PO 4 Mix it with 25ml of secondary ultrapure water) (continue dropwise for 2h), after the dropwise addition, reflux at 70°C to 76°C for 2h, cool the resulting reflux to 5°C to 20°C, filter, and wash the filter cake with water To neutral (pH=7), dry to constant weight at -0.08MPa~-0.09MPa, 70~80°C to obtain 12.95g of crude anthracene substituted at positions 9 and 10, the yield is 93.25%, and the HPLC purity is 99....
Embodiment 3
[0073] The difference from the above examples is that step 5 and the purification method in the preparation process are different, specifically:
[0074] Under the protection of argon, into a 500mL three-necked flask equipped with a mechanical stirrer, a condenser, and a thermometer, sequentially add (10-(1-naphthyl)anthracene-9-)boronic acid (10.0g), 2-(4-bromo Phenyl) naphthalene anthracene (18.31g), tetrahydrofuran (300ml), Pd-132 (0.020g), continue to heat up to 70 ° C ~ 76 ° C, continue to drop Cs 2 CO 3 Aqueous solution (Cs 2 CO 3 The aqueous solution consists of 89.37g Cs 2 CO 3 and 25ml of secondary ultrapure water) (continuously dropwise for 2h), after the dropwise addition, reflux at 70°C to 76°C for 2h, cool down to 5°C to 20°C, filter, and wash the filter cake to neutrality (pH = 7), dried to constant weight at -0.08MPa~-0.09MPa, 70~80°C to obtain 13.10 g of crude product of anthracene substituted at positions 9 and 10, with a yield of 93.85% and an HPLC purit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com