2-(2-alkoxy phenyl)-4, 5-diphenyl imidazole compound as well as synthesis method and application thereof
A technology of diphenylimidazole and alkoxyphenyl, applied in the field of 2--4,5-diphenylimidazole compounds and their synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Synthesis of 2-(4-butoxy-4'-(tert-butyl)-[1,1'-biphenyl]-3-yl)phenanthrene[9,10-d]imidazole (compound 1).
[0064]
[0065] Synthesis of 5-bromo-2-alkoxybenzaldehyde from 5-bromo-2-hydroxybenzaldehyde was accomplished according to the conventional method reported in the literature. References: Liu Jingzi, Wang Yuliang, Chen Shuhua, Journal of Southwest Normal University (Natural Science Edition), 2005, 30(2): 301-303; Li Jie, Tang Hongye, Xu Suoping, Jiangsu Normal University Journal (Natural Science Edition), 2012 , 30(3):43-46). Here, the reaction of 2-hydroxybenzaldehyde and 1-bromobutane is taken as an example. 20mmol (4.01g) of 5-bromo-2-hydroxybenzaldehyde, 40mmol (1.60g) of sodium hydroxide and 30mmol (4.11g) of bromobutane were added to 50mL of N,N-dimethylformamide. After stirring at room temperature for 5 hours, it was poured into 500 mL of water with stirring, the mixture was extracted with dichloromethane (50 mL×3 times), and the obtained organic layer ...
Embodiment 2
[0070] Synthesis of 2-(1-(2-butoxynaphthyl))-4,5-diphenylimidazole (compound 2).
[0071]
[0072]With reference to the reaction conditions of 5-bromo-2-hydroxybenzaldehyde and bromobutane in Example 1, 2-hydroxyl-1-naphthaldehyde and 1-bromobutane react to obtain 2-butoxyl-1-naphthaldehyde; With reference to the reaction conditions of 2-butoxyl-5-(4-tert-butyl)phenylbenzaldehyde and 9,10-phenanthrenequinone and ammonium acetate in Example 1 to synthesize imidazoles, the 2-butoxyl- The target compound 2 was synthesized from 1-naphthaldehyde, 1,2-diphenylethanedione and ammonium acetate, and the yield was 81% based on 2-butoxy-1-naphthaldehyde.
[0073] The NMR detection results of compound 2 are as follows: 1 H NMR (300MHz, CDCl 3 )δ: 9.35(d, J=8.7Hz, 1H), 8.70(d, J=7.8Hz, 2H), 7.81-7.74(m, 3H), 7.66-7.52(m, 7H), 7.42-7.39(m ,1H),7.23-7.19(m,2H),4.07(t,J=6.6Hz,2H),1.79-1.69(m,2H),1.48-1.38(m,2H),0.87(t,J=7.2 Hz, 3H). 13 C NMR (75MHz, CDCl3) δ: 155.0, 146.0, 132.9, 131....
Embodiment 3
[0075] Synthesis of 2-(1-(2-butoxynaphthyl))-phenanthrene[9,10-d]-imidazole (compound 3).
[0076]
[0077] Referring to Example 2, the target compound 3 was synthesized from 2-butoxy-1-naphthaldehyde, 9,10-phenanthrenequinone and ammonium acetate, and the yield was 86% based on 2-butoxy-1-naphthaldehyde.
[0078] The NMR detection results of compound 3 are as follows: 1 H NMR (300MHz, CDCl 3 )δ: 9.48(d, J=8.7Hz, 1H), 7.78(J=9.0Hz, 2H), 7.64-7.53(m, 4H), 7.41-7.22(m, 7H), 4.15(t, J=6.6 Hz, 2H), 1.82-1.75 (m, 2H), 1.49-1.42 (m, 2H), 0.91 (t, J=7.5Hz, 3H). 13 C NMR (75MHz, CDCl3) δ: 154.4, 142.8, 132.6, 130.7, 129.7, 128.6, 127.9, 127.5, 127.2, 127.0, 124.2, 114.2, 112.7, 69.9, 31.7, 19.5, 13.8. ESI-MS, m / z 417.2[M+H] + .
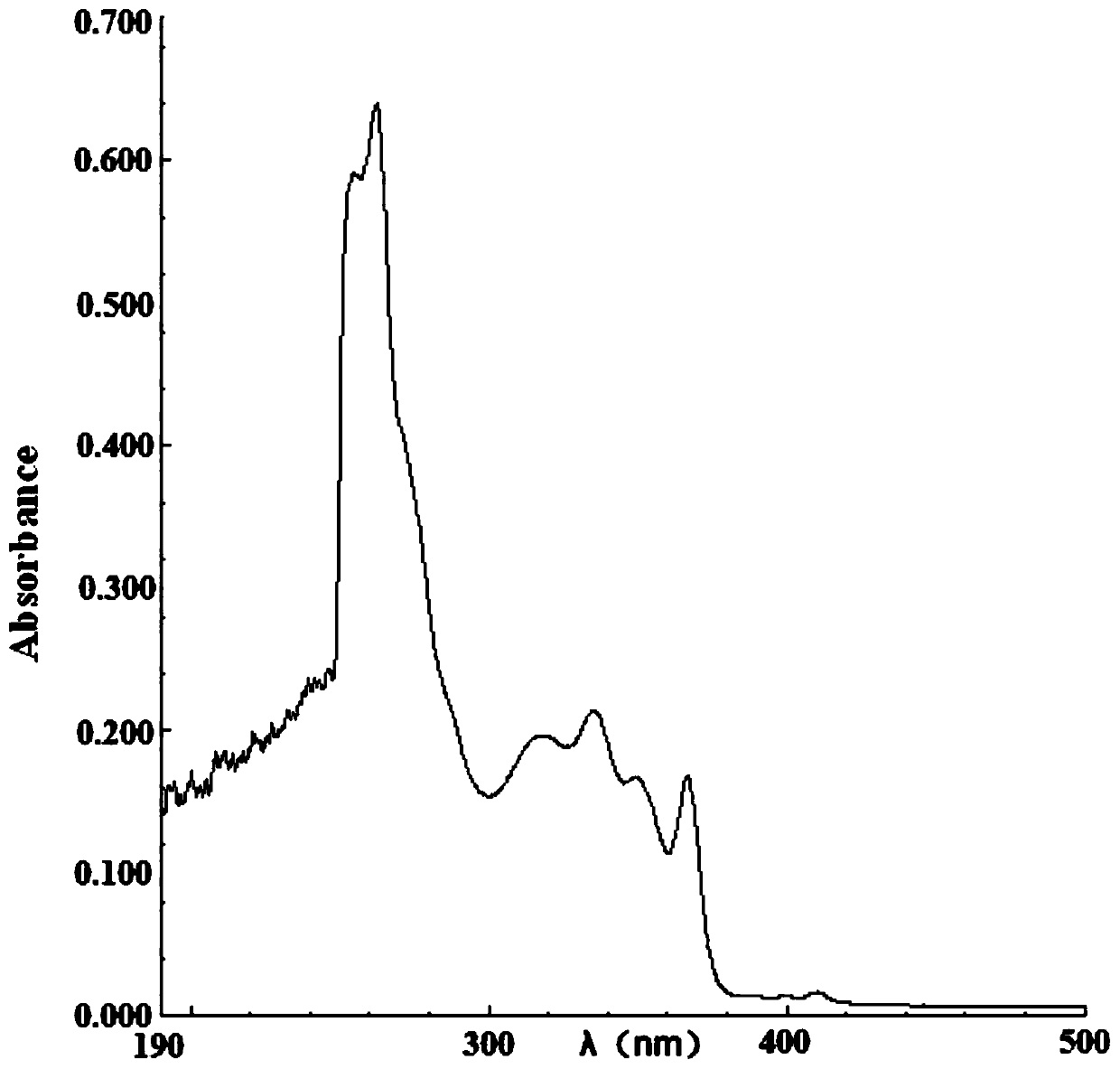
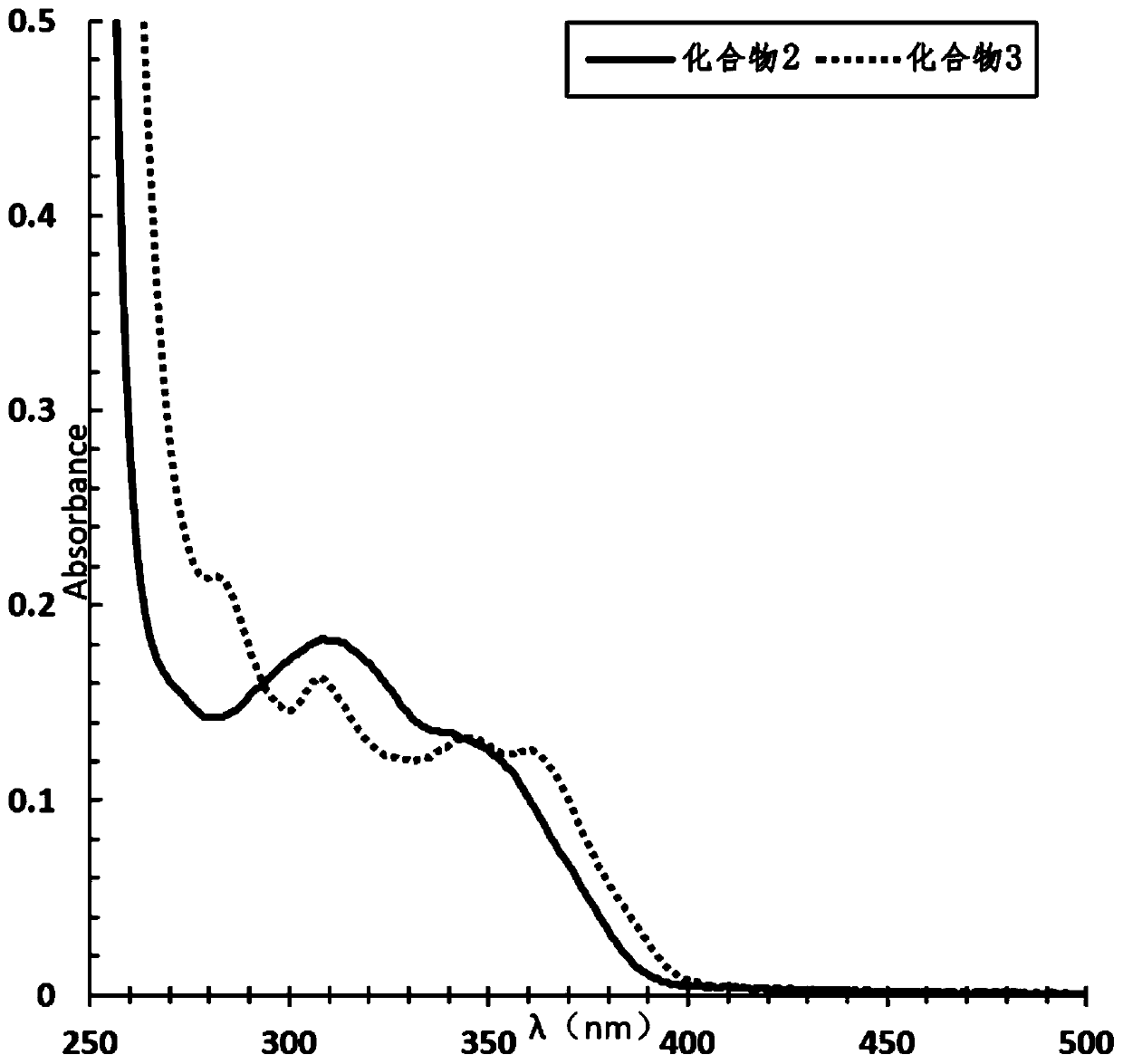
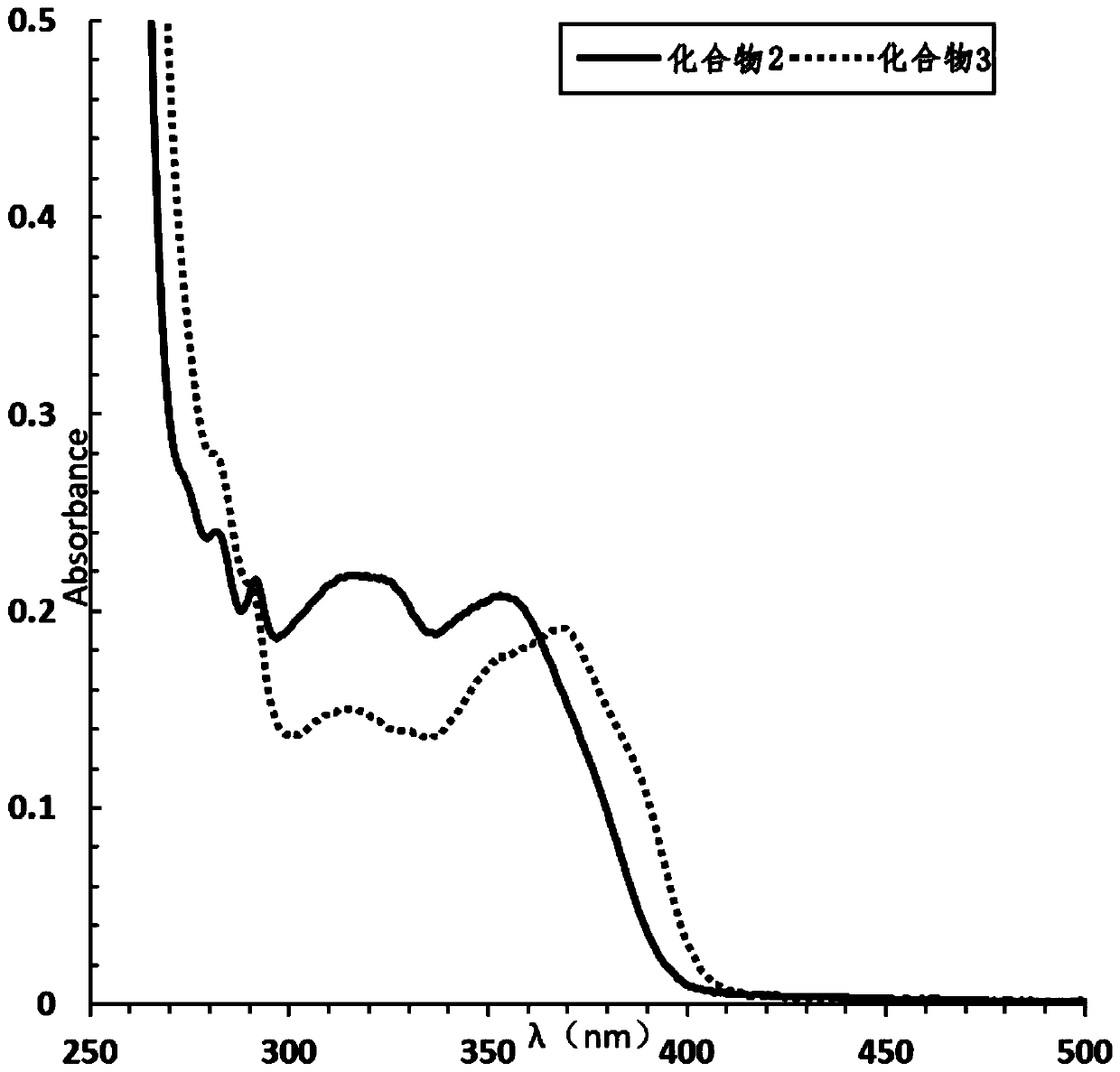
[0079] Performance Testing
[0080] The absorption properties of 2-(2-alkoxyphenyl)-4,5-diphenylimidazoles to ultraviolet light and blue light were detected.
[0081] Compound 1, compound 2 and compound 3 were dissolved in ethyl acetate and 1,2-dich...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com