Asymmetric quinoxaline acceptor unit material and polymer material by further copolymerization thereof, and application of asymmetric quinoxaline acceptor unit material and polymer material
A technology of polymer materials and acceptor units, which is applied in the field of organic synthesis and solar cells, can solve the problems of poor photoelectric conversion efficiency of Qx, and achieve the effect of improving photoelectric conversion efficiency and increasing solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
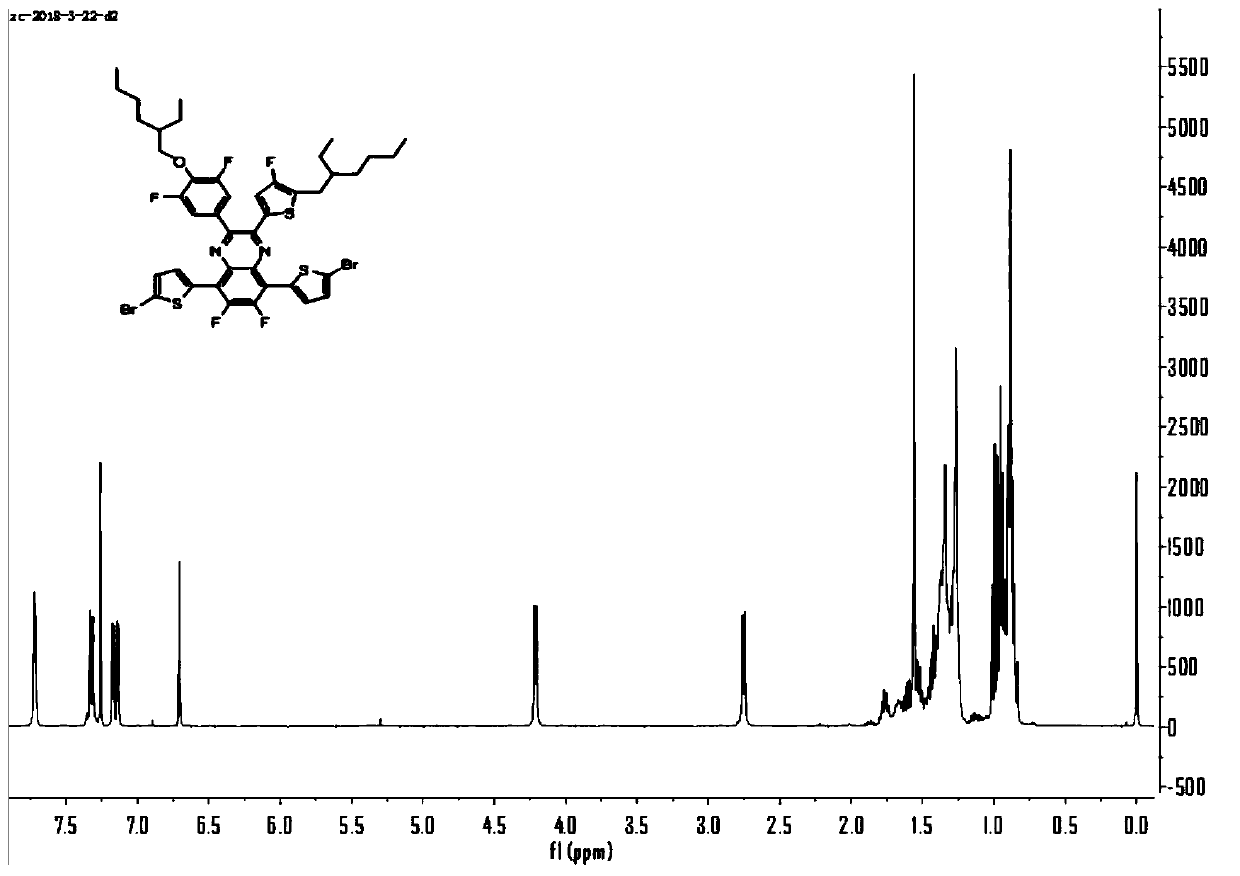
[0044] 1. Preparation of asymmetric quinoxaline acceptor material-1
[0045] 1) Compound 2 (15mmol), CuI (0.75mmol), Pd(PPh 3 ) 2 Cl 2 (0.3mmol), THF (60mL), NEt 3 (20mmol) was added into a 250ml three-neck flask, compound 1 (15mmol) was added under anaerobic conditions, and after reacting overnight at 80°C, the yield of compound 3 (6mmol) was 40%.
[0046]2) Compound 3 (6mmol), iodine (3mmol) and DMSO (50mL) were put into a 100ml round bottom flask, filled with Ar gas, and reacted overnight at 120°C to obtain compound 4 (4mmol) with a yield of 66.6%.
[0047] 3) Dissolve compound 5 (6mmol) and 60ml ethanol in a 250ml single-necked bottle, and react at 0°C; add sodium borohydride (30mmol) in batches, and stir at 0°C for 5.5h to obtain compound 6 (4.6mmol). rate of 76%.
[0048] 4) Put compound 6 (4.6mmol) and 100ml of acetic acid into a three-necked flask, put compound 4 (4mmol) and 60ml of acetic acid into a constant pressure dropping funnel, drop them in at 60°C under A...
Embodiment 2
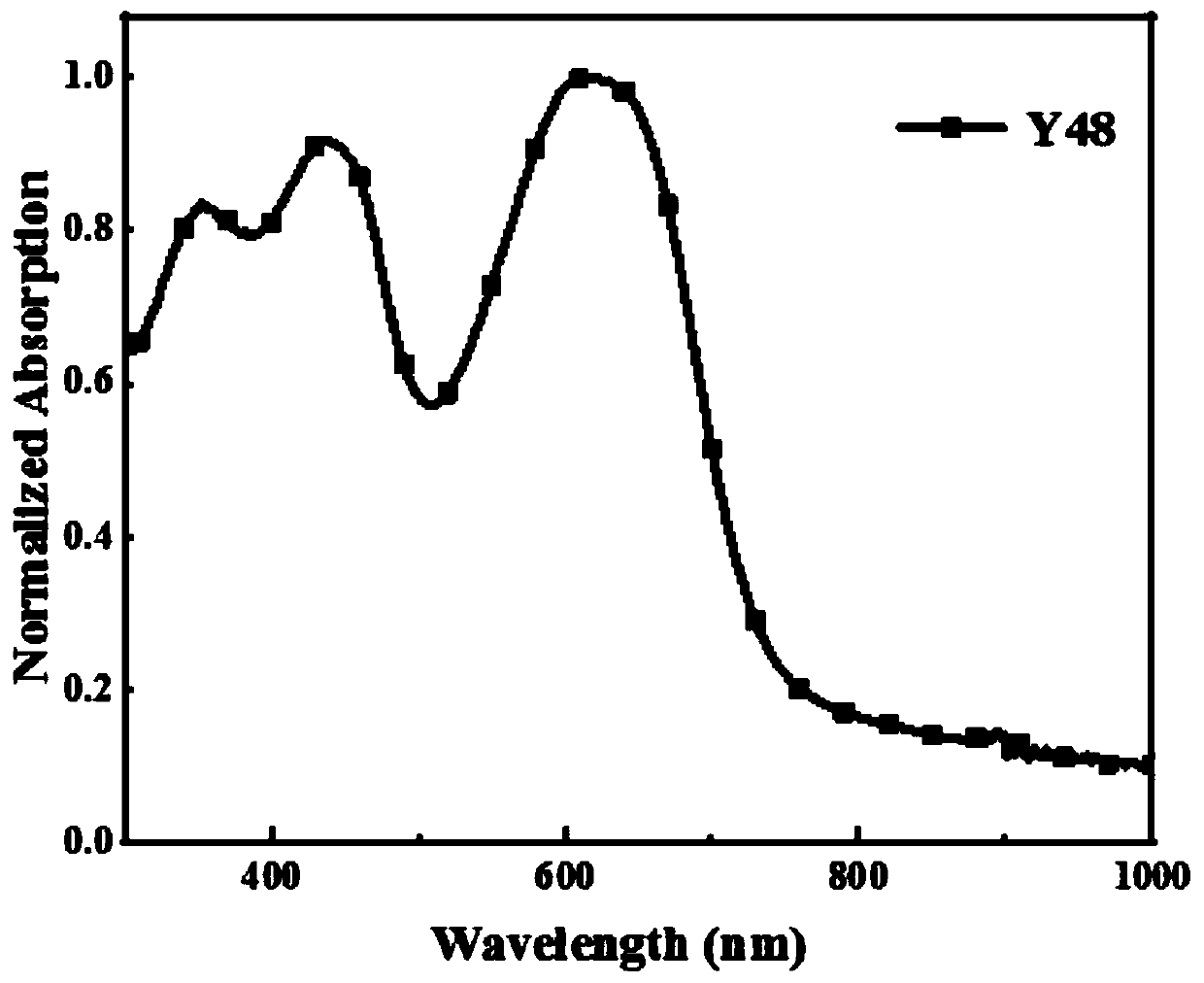
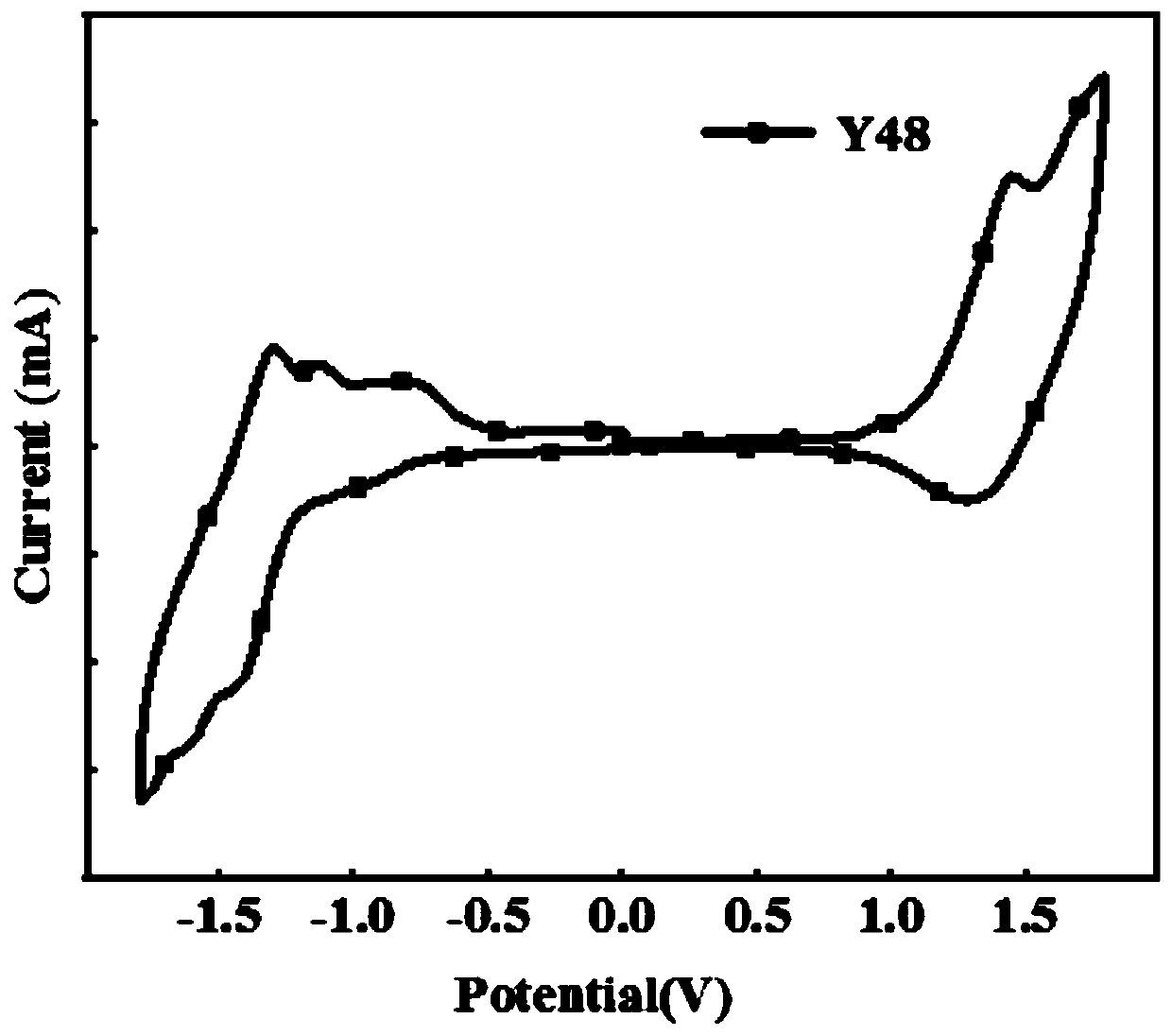
[0066] Photovoltaic performance of embodiment 2 Y48
[0067] The device structure is ITO / PEDOT:PSS / Y48:Y6 / PDINO / Ag
[0068] The photovoltaic performance test method of the copolymer Y48 donor material prepared in Example 1 is: Y48 and Y6 (electron acceptor material) are mixed with a mass ratio of 1:1.5 and dissolved in chloroform (wherein the concentration of Y48 is 6.4mg / uL), after fully dissolving, add chloronaphthalene with a volume ratio of 0.5%, spin-coat on the ITO conductive glass containing PEDOT:PSS interface, anneal at 100°C for 10 minutes, and then throw off 30 microliters of PDINO ethanol solution ; After preparing the sample, it is sent to the evaporation chamber and plated with 100 nanometer silver. The obtained device was tested in a solar simulator (A.M.1.5G, 100Mw / cm 2 ) on the test. Its photovoltaic performance is: V oc =0.79(V), J sc =20.93(mA / cm 2) , FF=65.92%, finally the optimal photoelectric conversion efficiency (PCE) is 10.4%.
[0069] The phot...
Embodiment 3
[0070] Photovoltaic performance of embodiment 3 Y49
[0071] The device structure is ITO / ZnO 2 / Y49:Y6 / MoO 3 / Ag
[0072] The photovoltaic performance test method of the copolymer Y49 donor material prepared in Example 1 is: Y49 and Y6 (electron acceptor material) are mixed with a mass ratio of 1:1.3 and dissolved in chloroform (wherein the concentration of Y48 is 6.4mg / μL), after fully dissolving, add chloronaphthalene with a volume ratio of 0.5%, spin-coat on the ITO conductive glass containing zinc oxide interface, and anneal at 110°C for 5 minutes. After the sample is prepared, it is sent to the evaporation chamber, coated with 12 nanometers of molybdenum oxide, and finally coated with 100 nanometers of silver. The obtained device was tested in a solar simulator (A.M.1.5G, 100Mw / cm 2 ) on the test. Its photovoltaic performance is: V oc =0.848(V),J sc =24.76(mA / cm 2) , FF=78.57%, finally the optimal photoelectric conversion efficiency (PCE) is 16.49%.
[0073] The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com