Method for predicting toxicity of chemicals taking zebra fish embryos as receptors by establishing QSAR model
A technology for prediction of zebrafish embryos and models, applied in chemical statistics, chemical machine learning, chemical property prediction, etc., can solve problems such as mechanism explanation, unsuitable for promotion and use, and large fluctuation of toxicity data quality, so as to achieve high-efficiency prediction Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
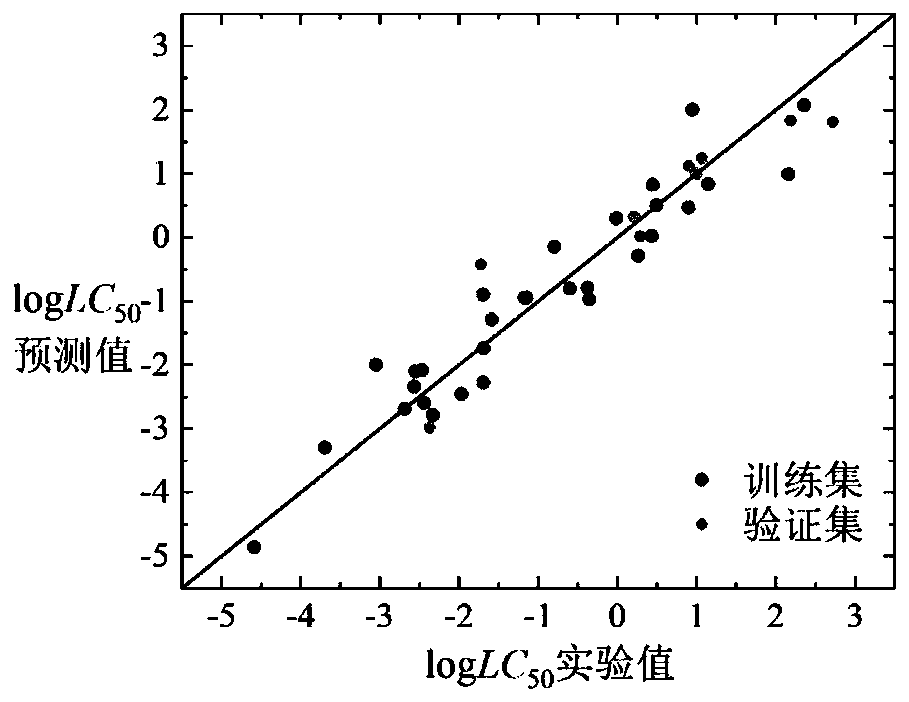
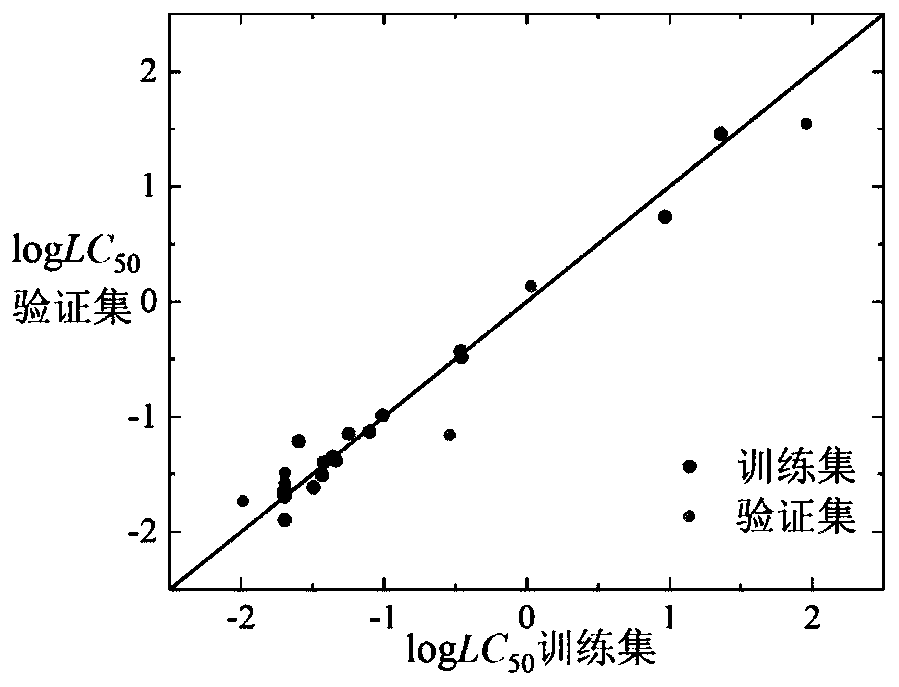
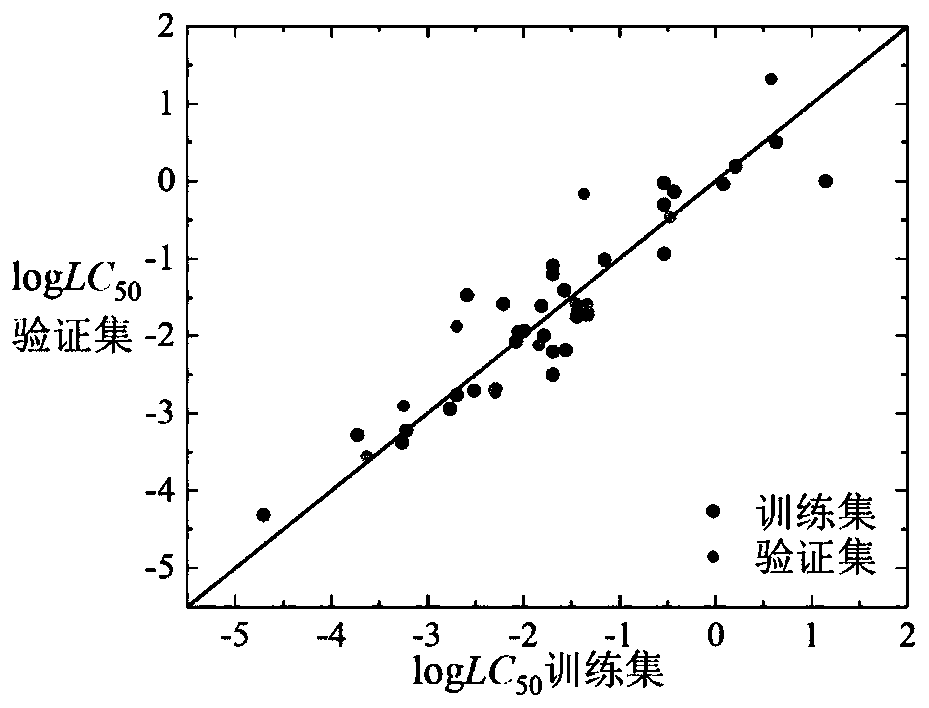
[0060] Given a compound isoamyl alcohol (CAS number: 123-51-3), to predict its logLC 50 value. First, according to the Smiles code of isoamyl alcohol, it is judged that it is an inert chemical substance, and its 3D structure is calculated using Openbabel software, and then its structure is optimized using PM6. Based on the optimized 3D structure, the descriptor is calculated using Dragon6.0 software corresponding value. The value of h calculated according to formula (7) is 0.087, attached Figure 7 The Williams diagram of the inert chemical substance in the medium shows that the leverage value (h) of this kind of substance is 0.5, and the leverage value of isoamyl alcohol is less than 0.5, so the compound is in the application domain of the model. Substituting the above descriptor values into the formula (1), we get logLC 50 The predicted value of 1.24, its experimentally determined logLC 50 The value is 1.06, and the predicted and experimental data are in good agreement...
Embodiment 2
[0062] Given a compound 2,4,5-trichlorophenol (CAS number: 95-95-4), to predict its logLC 50 value. First, according to the Smiles code of 2,4,5-trichlorophenol, it is judged that it is a weakly inert chemical substance, and its 3D structure is calculated using Openbabel software, and then its structure is optimized using PM6. Based on the optimized 3D structure, Draogon6 is used. 0 The software calculates the corresponding value of the descriptor. The h value calculated according to formula (7) is 0.147, with Figure 8 The Williams diagram of moderately weakly inert chemical substances shows that the leverage value (h) of such substances is 0.833. The leverage value of 2,4,5-trichlorophenol is less than 0.833, so this compound is within the application domain of the model, and the value of the above descriptor Substituting into formula (2), get logLC 50 The predicted value of -1.73, its experimentally determined logLC 50 With a value of -1.99, the predicted and experiment...
Embodiment 3
[0064]Given a compound cyhalofop-ethyl (CAS number: 122008-85-9), to predict its logLC 50 value. First, according to the Smiles code of cyhalofop-methyl, it is judged that it is an unclassifiable chemical substance, and its 3D structure is calculated using Openbabel software, and then its structure is optimized using PM6. Based on the optimized 3D structure, the descriptor is calculated using Draogon 6.0 software corresponding value of . According to the number of descriptors nRCOOR and nArCOOR, it is judged that it belongs to the chemical substances containing COOR in the chemical substances that cannot be classified. The value of h calculated according to formula (7) is 0.068, attached Figure 11 The Williams plot of the chemical species containing COOR in , shows that the leverage value (h) of such species is 0.5625. The leverage value of cyhalofop-ethyl is less than 0.5625, so the compound is in the application domain of the model, and the value of the above descriptor ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com