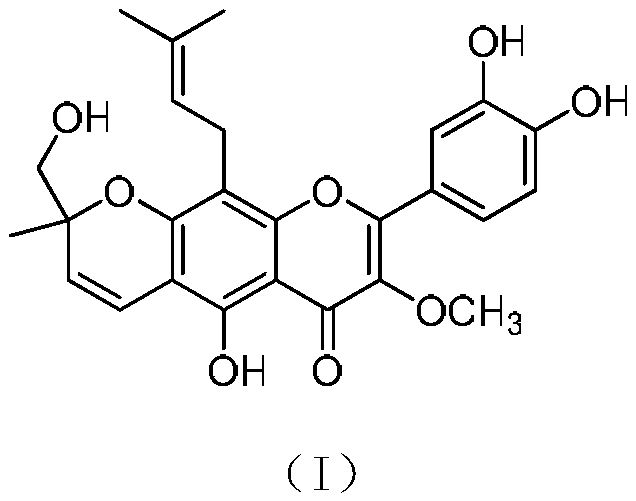
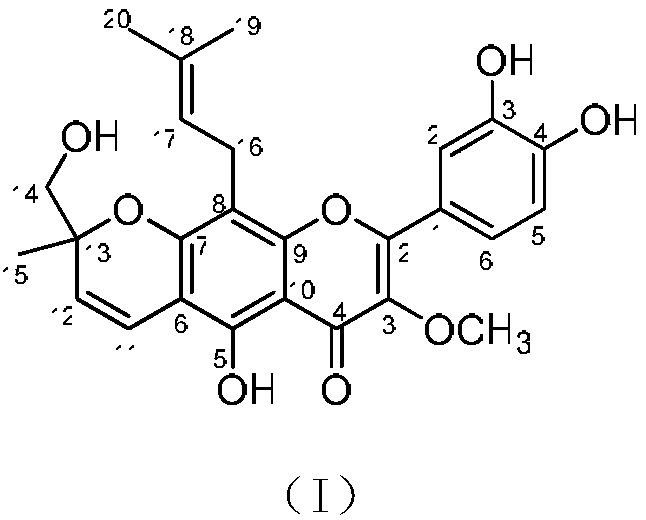
Compound extracted from broussometia kaempferi and application thereof in pharmacy
A technology of compounds and extracts, applied in the field of medicine, can solve the problems of unreported chemical composition and biological activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Embodiment 1 extracts compound of the present invention from rattan structure
[0017] (1) Extraction: after pulverizing 3.3 kg of rattan stems and branches, extract by percolation with 60 liters of 95% ethanol, collect the percolation liquid, concentrate under reduced pressure to obtain 100 grams of extract. The extract was dissolved in water and extracted with ethyl acetate, and the recovered solvent was concentrated to dryness to obtain 74 grams of ethyl acetate extract;
[0018] (2) Separation: 74 grams of ethyl acetate extract was subjected to MCI gel CHP-20P column chromatography, and methanol-water (50:50→60:40→70:30→80:20→90:10→100: 0) Gradient elution, the amount of each gradient is 15 liters, and the fractions are collected; wherein the methanol-water 90:10 fraction (8 grams) is subjected to silica gel column chromatography, and petroleum ether-acetone (30:1~1:1) Gradient elution, 2 liters of each gradient solvent consumption, thin-layer chromatography detect...
Embodiment 2
[0020] Example 2 Determination of the inhibitory activity of the compound (I) of the present invention to protein tyrosine phosphatase 1B (protein tyrosinephosphatase 1B, PTP1B)
[0021] (1) Preparation of protein tyrosine phosphatase 1B protein
[0022]The recombinant plasmid containing the PTP1B catalytic domain was transformed into Escherichia coli BL21-CodonPlus (DE3) for expression; the BL21-CodonPlus (DE3) cells containing the recombinant plasmid were inoculated in Luria-Bertani containing ampicillin (ampicillin) 100mg / l On (LB) medium, shake culture at 37°C, add isopropylthiogalactopyranoside (IPTG) to 500nM to induce protein expression when the cell concentration reaches the absorbance value at 600nm between 0.4-0.6; use GSTrap FF column was used for protein sample purification; 10% polyacrylamide gel electrophoresis was used for protein analysis, and the protein concentration was determined by the Coomassie brilliant blue method (Bradford), with bovine serum albumin (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com