Pharmaceutical composition containing carbohydrate nutrient and conventional ineffective compound and application of pharmaceutical composition
A nutrient and composition technology, applied to the device comprising the composition, the field of topical pharmaceutical compositions for the treatment of local pathological diseases, to achieve the effects of non-toxic systemic safety, low local irritation, and effective treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0090] According to the preparation method of the present invention, the preparation of the pharmaceutical composition of the present invention comprises the following steps: preparing a liquid medicine containing the topical active ingredient, a liquid medium and other substances optionally present. The liquid medicament may be a solution (eg, in a hydrophilic vehicle, preferably an aqueous solution), a suspension, or an emulsion containing the topically active ingredient. When the liquid drug is a suspension, the dispersion medium therein can be any suitable one known to those skilled in the art, such as micro-materials or nano-materials. When the liquid drug is an emulsion, the dispersion medium therein can be any suitable one known to those skilled in the art, such as vegetable oil, synthetic oil or semi-synthetic oil that can be used for injection. Wherein said vegetable oil may be, for example, cottonseed oil, almond oil, olive oil, castor oil, sesame oil, soybean oil an...
Embodiment 1
[0179] Embodiment 1: the preparation of composition of the present invention
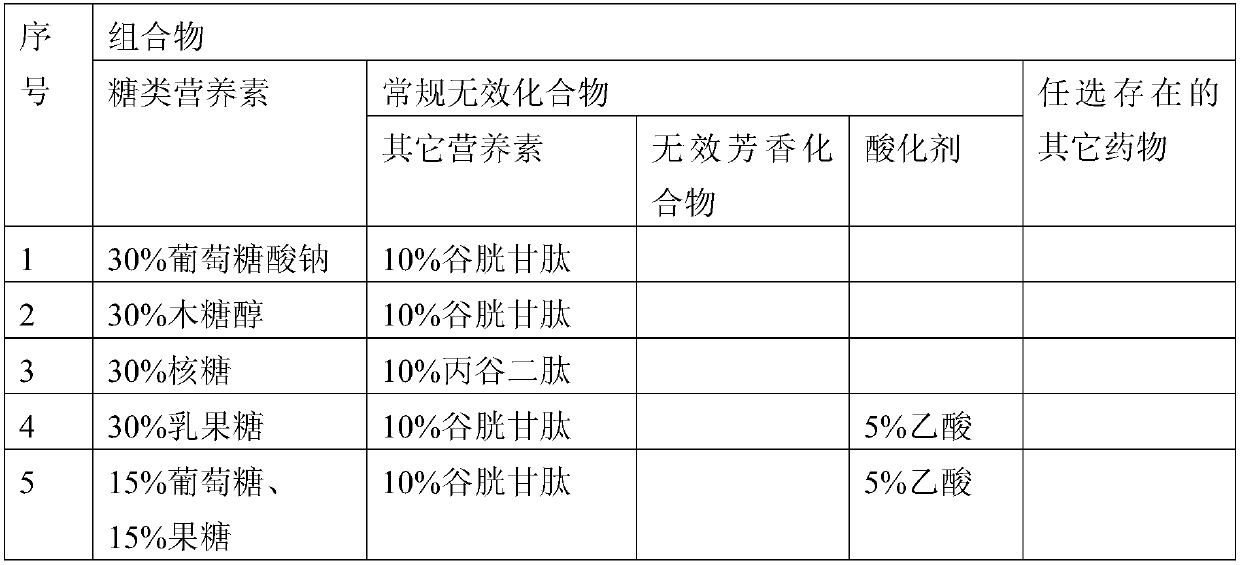
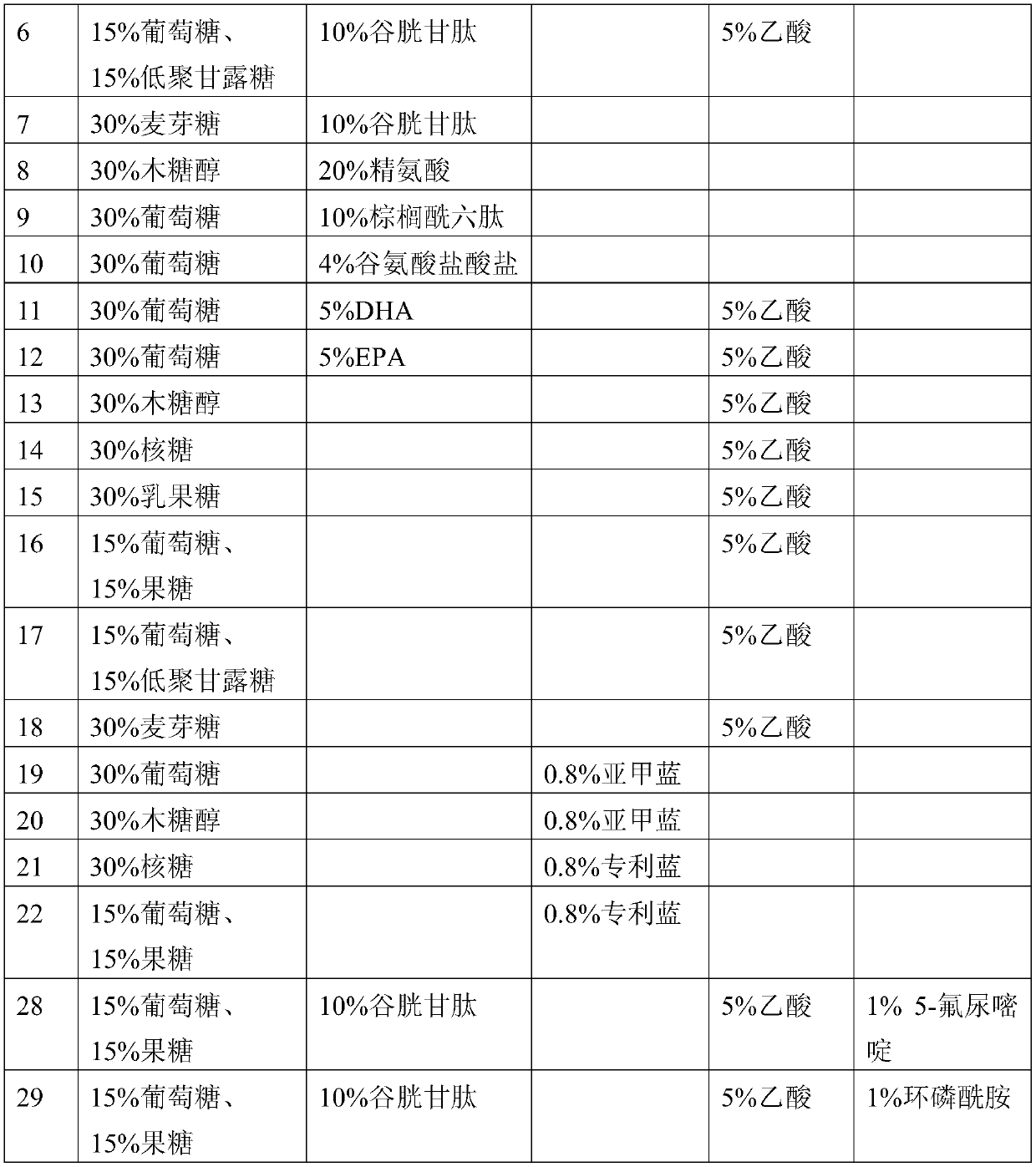
[0180] According to the above preparation method of the composition of the present invention, many different compositions of the present invention can be prepared. The compositions of some compositions of the present invention prepared in this example are listed in Table 2.
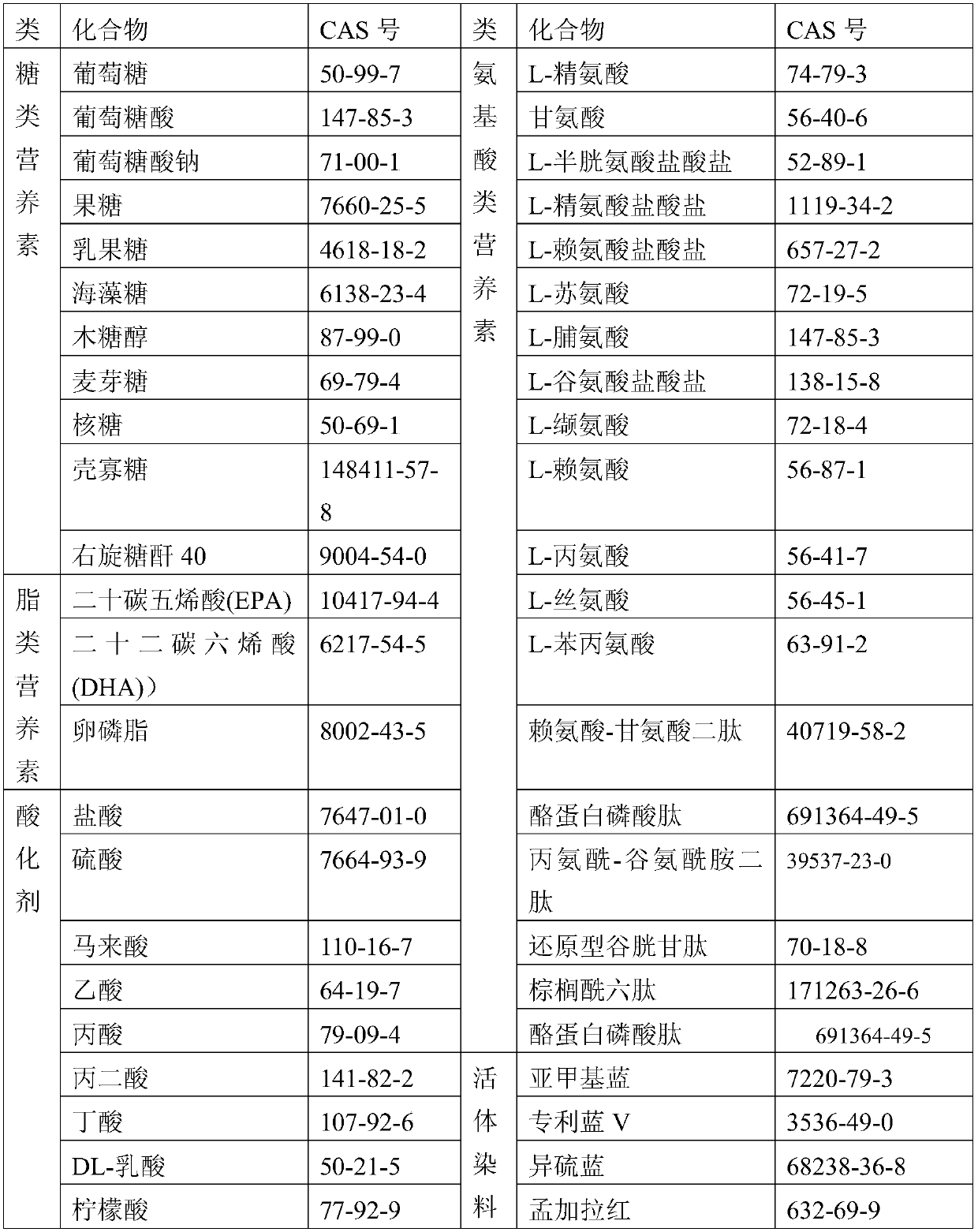
[0181] Table 2
[0182]
[0183]
[0184] Several examples of preparation tests of the compositions of the present invention are listed below.
[0185] 1. Preparation of liquid injection (1)
[0186] Measure glyconutrients (such as 30g glucose), conventional invalid compounds (such as 10g glutathione), optional other components and dilute to total volume (such as 100ml) according to the required concentration (as described in Table 2). ) solvent (such as water for injection), and they are slowly mixed evenly, and after sterilizing and filtering, they are subpackaged into required quantities (such as 10ml / bottle) and sto...
Embodiment 2
[0199] Embodiment 2: Composition synergistic technical scheme is preferred
[0200] 1. Synergistic pharmacological optimization
[0201] In this research experiment, the successfully modeled experimental animals (bearing S180 mice, with an average tumor volume of 115mm 3) were randomly divided into 2 negative control groups and 32 research groups. The negative control was physiological saline, and the 16 research drugs were shown in the table below, and they were injected intraperitoneally and intratumorally respectively. The medicines are all aqueous solutions, which are prepared according to the preparation method of Example 1. Medicate once every 3 days, a total of 3 times, each time 115μl / only. The animals were euthanized on the next day after the administration, and the tumor weight was measured after dissection, and the tumor inhibition rate was calculated from the respective negative control groups. The results are shown in Table 3.
[0202] table 3
[0203] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com