N-bisbenzenesulfonyl-1-phenylseleno trifluoroethane derivative, synthesis method and applications thereof
A technology of phenylselenyltrifluoroethane and bisphenylsulfonyl, applied in the field of N-bisphenylsulfonyl-1-phenylselenyltrifluoroethane derivatives and their synthesis, can solve the problem of many active functional groups and difficult synthesis , No synthetic method and other problems were found, achieving the effect of simple and easy-to-obtain raw materials, mild conditions, and high-efficiency atom economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
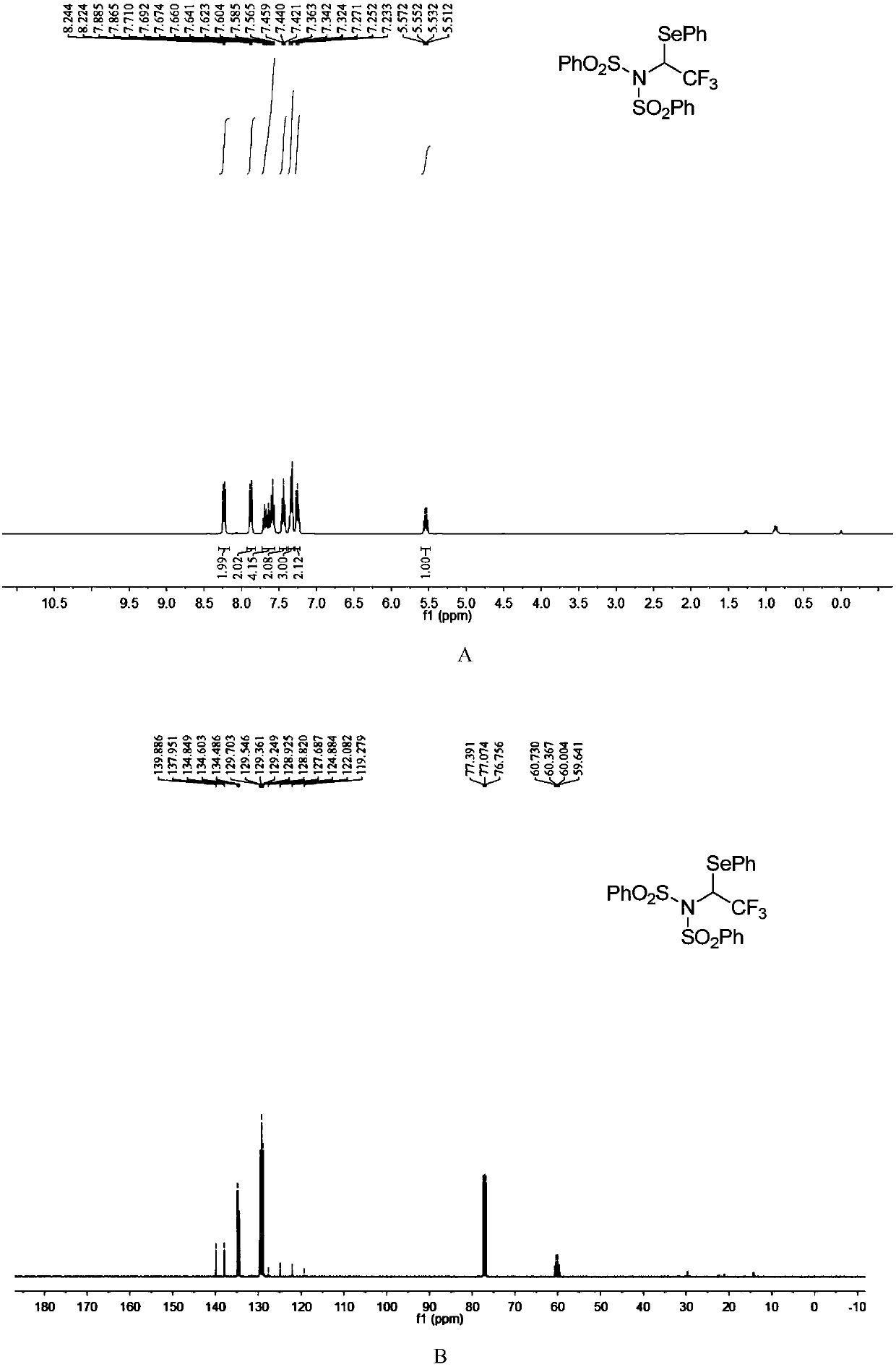
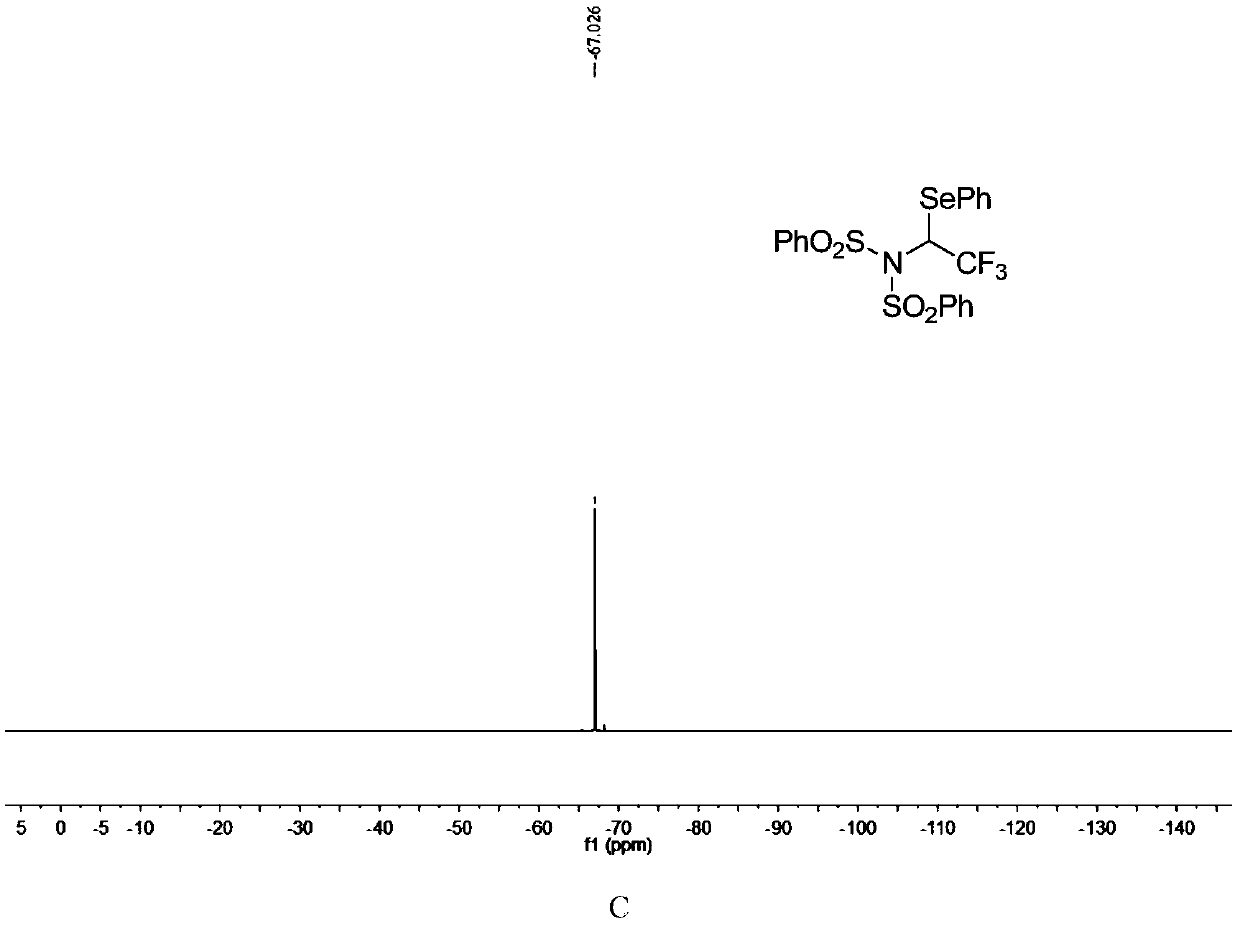
[0051]
[0052] N-fluorobisbenzenesulfonimide (0.2mmol), diphenyldiselenide (0.12mmol) were dissolved in dichloromethane (2.0mL), then, diluted in dichloromethane (1mL) of trifluoromethane The toluene solution (c=0.85mmol / mL, 0.4mmol) of diazonium was slowly added dropwise to the reaction system, and the reaction system was at room temperature (25°C). After the dropwise addition was completed, it was stirred for 10 minutes, and the solvent was removed under reduced pressure to obtain The crude product has a structure as shown in formula (4-1). The crude product was subjected to column chromatography (ethyl acetate:petroleum ether=1:40~1:20) to obtain a pure product. The yield was 95%. nuclear magnetic resonance 1 H NMR, 13 C NMR, 19 F NMR spectrum such as figure 1 as shown, 1 H NMR (400MHz, CDCl 3 ):δ8.23(d,J=8.2Hz,2H),7.87(d,J=8.2Hz,2H),7.71-7.57(m,4H),7.46-7.42(m,2H),7.36-7.32( m,3H),7.27-7.23(m,2H),5.54(q,J=8.0Hz,1H)ppm; 13 CNMR (100MHz, CDCl 3 ): δ139.9, 138.0...
Embodiment 2
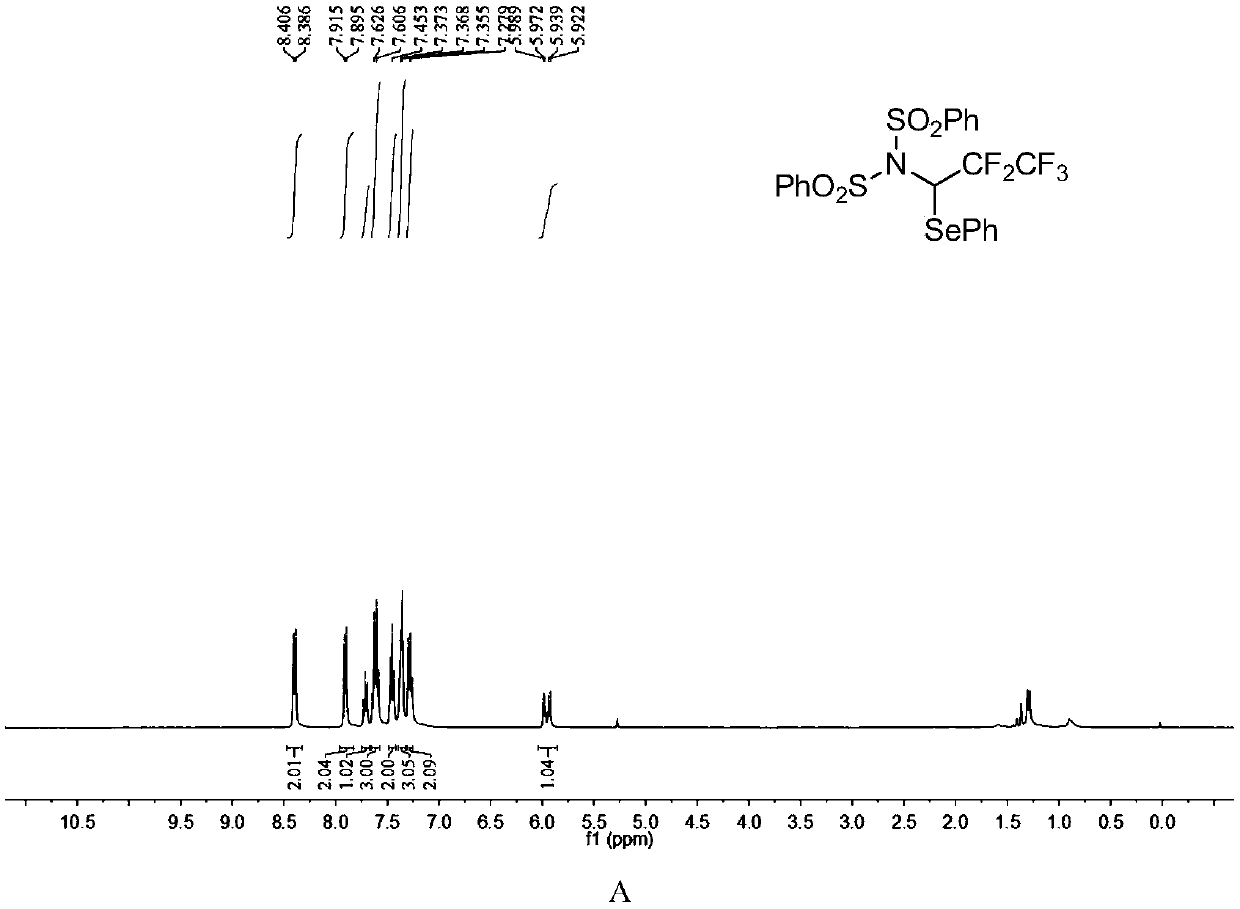
[0054]
[0055] N-fluorobisbenzenesulfonylimide (0.2mmol), diphenyldiselenide (0.12mmol) were dissolved in dichloromethane (2.0mL), and then, diluted in dichloromethane (1mL) pentafluoroethyl The toluene solution (c=0.85mmol / mL, 0.4mmol) of diazonium was slowly added dropwise to the reaction system, and the reaction system was at room temperature (25°C). After the dropwise addition was completed, it was stirred for 10 minutes, and the solvent was removed under reduced pressure to obtain The crude product has a structure as shown in formula (4-2). The crude product was subjected to column chromatography (ethyl acetate:petroleum ether=1:40~1:20) to obtain a pure product. The yield was 88%. nuclear magnetic resonance 1 H NMR, 13 C NMR, 19 F NMR spectrum such as figure 2 as shown, 1 H NMR (400MHz, CDCl 3 ): δ8.40(d, J=7.8Hz, 2H), 7.90(d, J=7.9Hz, 2H), 7.71(t, J=7.4Hz, 1H), 7.62(q, J=7.8Hz, 3H ), 7.45 (t, J = 7.8Hz, 2H), 7.40-7.32 (m, 3H), 7.31-7.25 (m, 2H), 5.96 (dd, J...
Embodiment 3
[0057]
[0058] N-fluorobisbenzenesulfonylimide (0.2mmol), diphenyldiselenide (0.12mmol) were dissolved in dichloromethane (2.0mL), then difluoromethane diluted in dichloromethane (1mL) A chloroform solution of diazo (c=0.85mmol / mL, 0.4mmol) was slowly added dropwise to the reaction system, and the reaction system was at room temperature (25°C). After the dropwise addition was completed, it was stirred for 10 minutes, and the solvent was removed under reduced pressure to obtain The crude product has a structure as shown in formula (4-3). The crude product was subjected to column chromatography (ethyl acetate:petroleum ether=1:40~1:20) to obtain a pure product. The yield was 85%. nuclear magnetic resonance 1 H NMR, 13 C NMR, 19 F NMR spectrum such as image 3 as shown, 1 H NMR (400MHz, CDCl 3 ): δ8.12(d, J=7.6Hz, 2H), 7.70(d, J=7.6Hz, 2H), 7.59-7.41(m, 5H), 7.29(d, J=7.9Hz, 3H), 7.20 (t, J=7.3Hz, 1H), 7.12(t, J=7.4Hz, 2H), 6.44-6.13(m, 1H), 5.23-5.11(m, 1H)ppm; 13 C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com