A kind of assay method of Qianlieping capsule
A technology of Qianlieping capsule and determination method, applied in the field of pharmaceuticals, can solve the problem of not formulating other ingredients and the like, and achieve the effects of stable and controllable quality, good accuracy and guaranteed curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment one: prescription and preparation method of Qianlieping Capsules
[0028] prescription:
[0029] Beibaijiang 702g, Danshen 234g, Chishao 234g, Peach Kernel 234g, Safflower 234g
[0030] Zeilan 234g Shiwei 234g Frankincense 47g Myrrh 47g
[0031] Preparation method: the above nine flavors, frankincense and myrrh are crushed into fine powder and set aside. Grind Danshen and Radix Paeoniae Rubra into coarse powder, add ethanol to extract twice, each time for 2 hours, combine the extracts, filter, recover ethanol from the filtrate and concentrate to a thick paste with a relative density of 1.32-1.36 (60°C), and set aside. The other five flavors such as Beibaijiang and the medicinal residue after alcohol extraction are decocted twice, the first time is 2 hours, the second time is 1.5 hours, the decoction is combined, filtered, and the filtrate is concentrated under reduced pressure to a relative density of 1.32-1.36 ( 60°C), add the above-mentioned fine powder ...
Embodiment 2
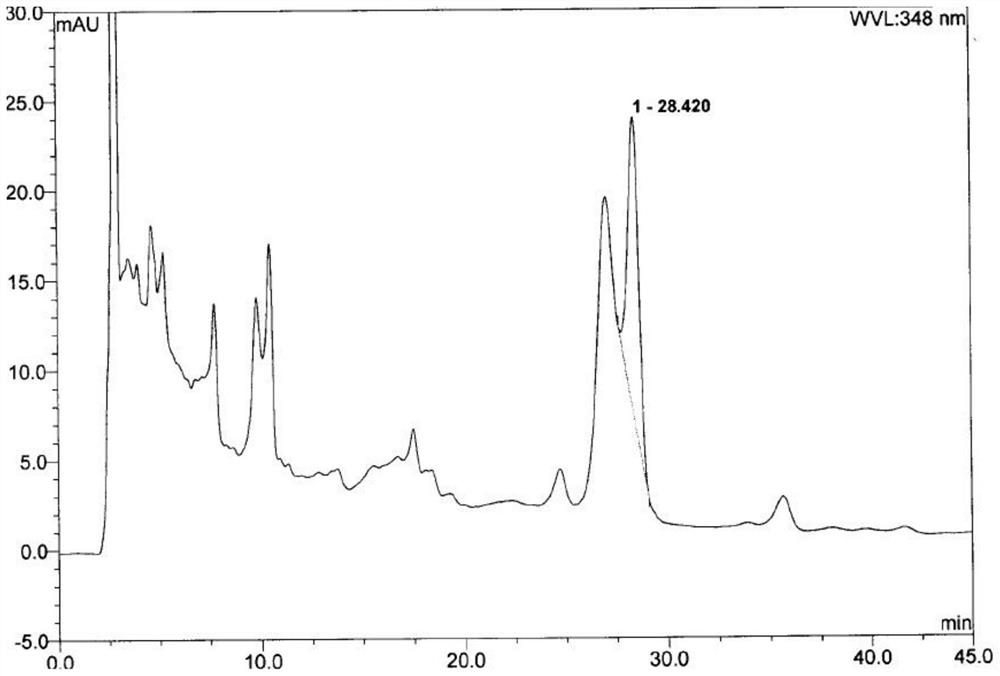
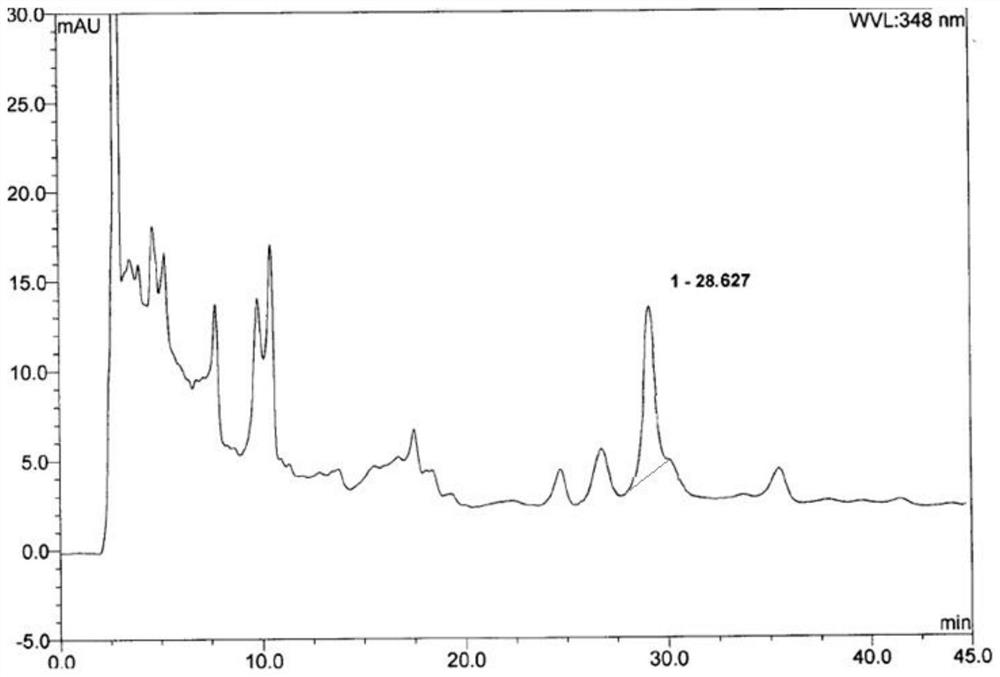
[0033] Example 2: Content identification of luteolin in Beibaijiang in Qianlieping capsules
[0034] 1. Optimum test of mobile phase and preparation method of test product
[0035] The content of the active ingredient luteolin in Beibaijiang in Qianlieping capsules was determined by HPLC, wherein the chromatographic conditions were:
[0036] Packing agent: octadecylsilane bonded silica gel; column temperature: 35°C; use a UV detector, the detection wavelength is preferably 348nm; the number of theoretical plates should not be less than 4000 based on the luteolin peak. The flow rate was 1 ml / min.
[0037] Mobile phase optimization selection comparison:
[0038] Mobile phase 1: The mobile phase is methanol-water solution with a volume ratio of 30:70.
[0039] Mobile phase 2: The mobile phase is acetonitrile-1.0% (v / v) formic acid solution in a volume of 30:70.
[0040] The following sample processing methods were combined for experimentation:
[0041] The high-performance l...
Embodiment 3
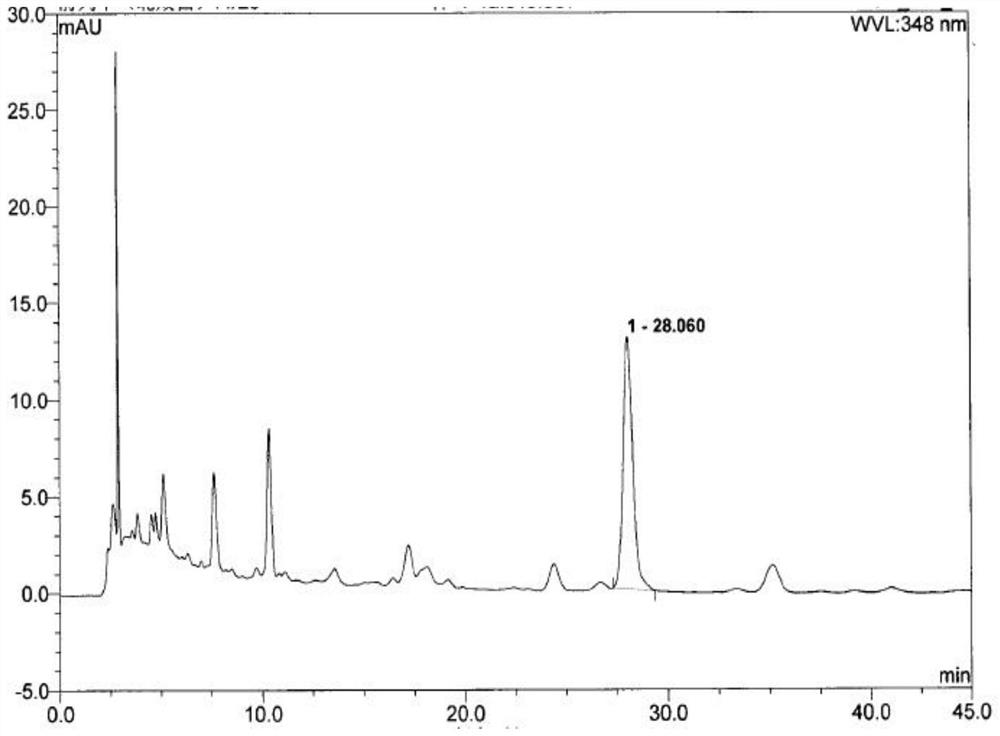
[0121] Example 3: Content Determination and Stability Test of Luteolin in Beibaijiang in Qianlieping Capsules
[0122] The samples produced by Qianlie Pingda (batch numbers: 20180101, 20180102, 20180103, produced by Xi'an Qianhe Pharmaceutical Co., Ltd.) were subjected to accelerated experiments and room temperature placement experiments. Accelerated experiment: Place the sample in a closed environment with a relative humidity of 75% ± 5% and a temperature of 40°C ± 2°C for 6 months. In the 0th, 1st, 2nd, 3rd, and 6th months, samples were taken for character observation. Limit detection; room temperature placement test: placed in a closed environment with a relative humidity of 60% ± 5% and a temperature of 25 °C ± 2 °C for 3 months, at the 0th, 3rd, 6th, 12th, and 18th months, samples were taken for inspection, and the test results are shown in the table 6. The test only retained the content data of luteolin.
[0123] The stability test data table of luteolin content in Tabl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com