Preparation method and application of chalcone derivative QNL-Chalcone
A technology of chalcone derivatives and ethyl ketone, which is applied in drug combinations, nervous system diseases, organic chemistry, etc., can solve the problems of strong antidepressant activity, insufficient molecular activity, and long synthetic route, and achieve low price and easy scale-up. The effect of large-scale industrial mass production and short synthetic route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] A preparation method and application of a chalcone derivative QNL-Chalcone, comprising the following steps:
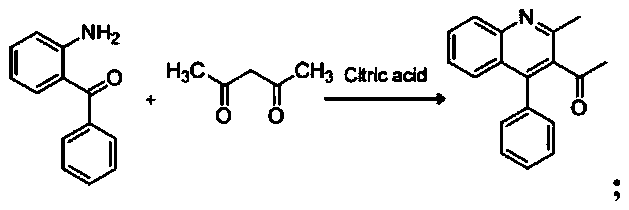
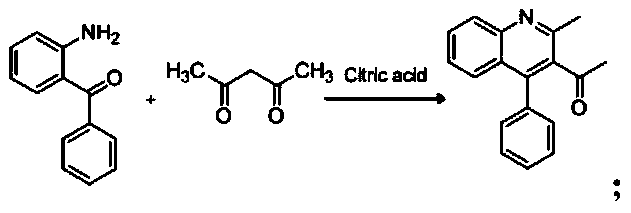
[0037] (1), the synthesis of 1-(2-methyl-4-phenylquinolin-3-yl)ethanone:
[0038]
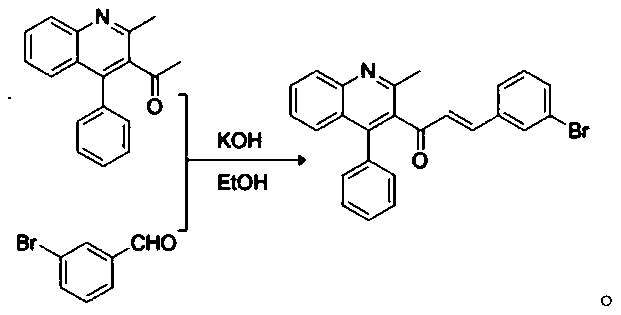
[0039] (2), the synthesis of QNL-Chalcone:
[0040]
[0041] The specific process of the step (1) is: take 4.8 mmol of acetylacetone and 2.1 mmol of citric acid in a round-bottomed flask, stir and reflux on a magnetic stirrer at a temperature of 102 ° C, and mix 4.3 mmol of 2-amino di Benzophenone was dissolved in 16ml of 1,4-dioxane in a constant-pressure dropping funnel, and slowly dropped into a round-bottomed flask under stirring conditions, and the reaction progress was monitored by TLC. After the reaction, add 2mol KOH solution to the reaction system, extract 3 times with 31ml ethyl acetate, wash with water until neutral, anhydrous Na 2 SO 4 Dry, filter with suction, spin dry, and recrystallize from ethanol to obtain compound 1-(2-methyl-4-phenylquinolin-3-yl)ethan...
Embodiment 2
[0058] The difference from Example 1 is that the specific process of the step (1) is: take 4.8 mmol of acetylacetone and 2 mmol of citric acid in a round-bottomed flask, stir and reflux on a magnetic stirrer at a temperature of 95° C., and 4mmol of 2-aminobenzophenone was dissolved in 15ml of 1,4-dioxane in a constant-pressure dropping funnel, slowly dropped into a round-bottomed flask under stirring conditions, and the reaction progress was monitored by TLC. After the reaction, add 1.9mol KOH solution to the reaction system, extract 3 times with 30ml ethyl acetate, wash with water until neutral, anhydrous Na 2 SO 4 Dry, filter with suction, spin dry, and recrystallize from ethanol to obtain compound 1-(2-methyl-4-phenylquinolin-3-yl)ethanone (1a).
[0059] The specific process of step (2) is: get 2mmol of 1-(2-methyl-4-phenylquinolin-3-yl)ethanone (1a), 2.2mmol of 2-bromobenzaldehyde in a round bottom flask, add 28ml of 4% KOH ethanol solution was stirred at room temperatur...
Embodiment 3
[0061] The difference from Example 1 is that the specific process of the step (1) is: take 4.8 mmol of acetylacetone and 2.2 mmol of citric acid in a round bottom flask, stir and reflux on a magnetic stirrer at a temperature of 110 ° C, Dissolve 4.5 mmol of 2-aminobenzophenone in 18 ml of 1,4-dioxane in a constant-pressure dropping funnel, slowly drop into a round-bottomed flask under stirring, and monitor the reaction progress by TLC. After the reaction, add 2.1mol KOH solution to the reaction system, extract 3 times with 32ml ethyl acetate, wash with water until neutral, anhydrous Na 2 SO 4 Dry, filter with suction, spin dry, and recrystallize from ethanol to obtain compound 1-(2-methyl-4-phenylquinolin-3-yl)ethanone (1a).
[0062] The specific process of step (2) is: get 2mmol of 1-(2-methyl-4-phenylquinolin-3-yl)ethanone (1a), 2.6mmol of 2-bromobenzaldehyde in a round bottom flask, add 32ml of 4.5% KOH ethanol solution was stirred at room temperature, and the reaction pr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com